Label: CETIRIZINE HYDROCHLORIDE tablet, film coated
- NDC Code(s): 0378-3635-01, 0378-3637-01, 0378-3637-05
- Packager: Mylan Pharmaceuticals Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 12, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
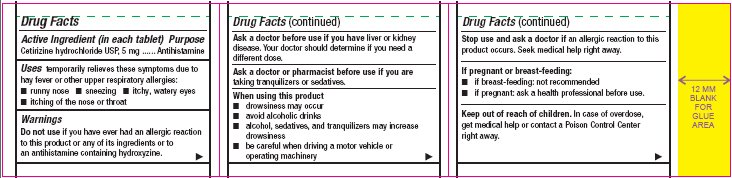
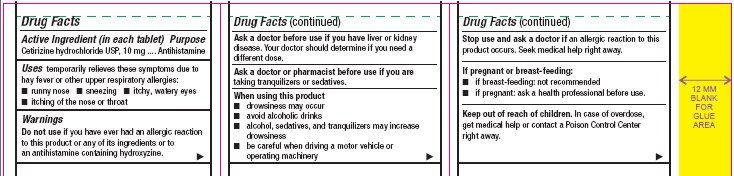
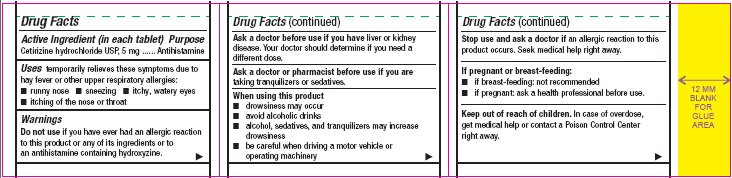
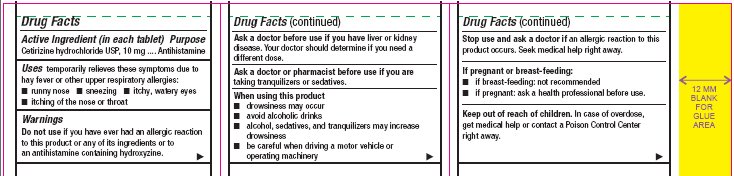
- Active Ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Do not use
if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
Ask a doctor before use if you have
liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
- •
- drowsiness may occur
- •
- avoid alcoholic drinks
- •
- alcohol, sedatives, and tranquilizers may increase drowsiness
- •
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if
an allergic reaction to this product occurs. Seek medical help right away.
-
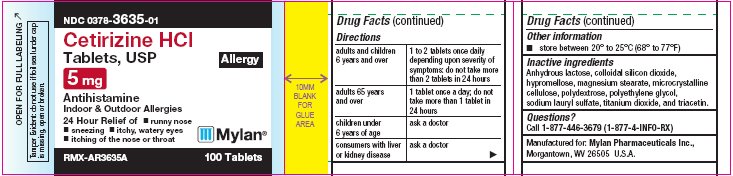
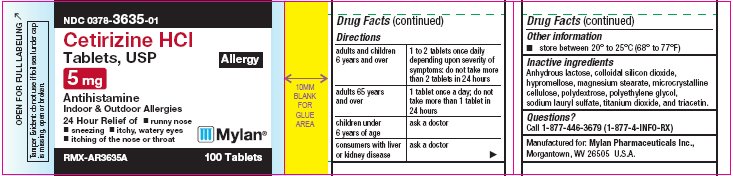
Directions
adults and children 6 years and over
1 to 2 tablets once daily depending upon severity of symptoms; do not take more than 2 tablets in 24 hours.
adults 65 years and over
1 tablet once a day; do not take more than 1 tablet in 24 hours
children under 6 years of age
ask a doctor
consumers with liver or kidney disease
ask a doctor
- Other information
- Inactive ingredients
- Questions?
-
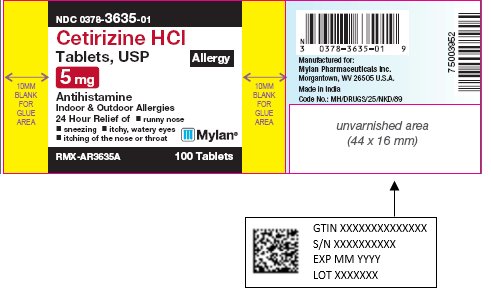
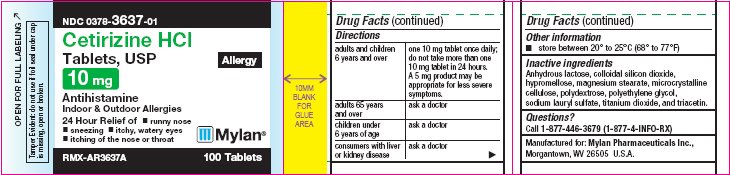
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 5 mg Allergy
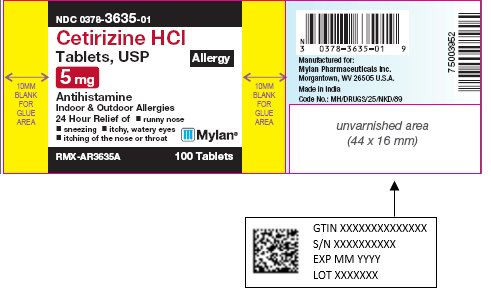
NDC 0378-3635-01
Cetirizine HCl
Tablets, USP
Allergy
5 mg
AntihistamineIndoor & Outdoor Allergies
24 Hour Relief of
- •
- runny nose
- •
- sneezing
- •
- itchy, watery eyes
- •
- itching of the nose or throat
RMX-AR3635A 100 Tablets
Tamper Evident: do not use if foil seal
under cap is missing, open or broken. -
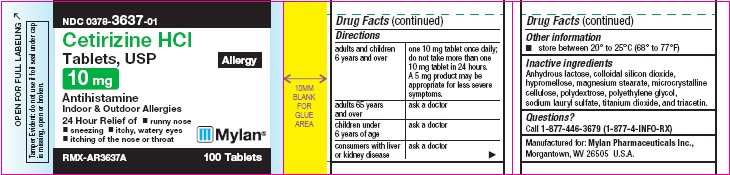
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 10 mg Allergy
NDC 0378-3637-01
Cetirizine HCl
Tablets, USP
Allergy
10 mg
AntihistamineIndoor & Outdoor Allergies
24 Hour Relief of
- •
- runny nose
- •
- sneezing
- •
- itchy, watery eyes
- •
- itching of the nose or throat
RMX-AR3637A 100 Tablets
Tamper Evident: do not use if foil seal
under cap is missing, open or broken.Active Ingredient (in each tablet)
Cetirizine hydrochloride USP, 10 mgDirections
adults and children 6 years and over
one 10 mg tablet once daily; do not take more than one 10 mg tablet in 24 hours.
A 5 mg product may be appropriate for less severe symptoms.
adults 65 years and over
ask a doctor
children under 6 years of age
ask a doctor
consumers with liver or kidney disease
ask a doctor
-
INGREDIENTS AND APPEARANCE
CETIRIZINE HYDROCHLORIDE
cetirizine hydrochloride tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0378-3635 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE HYDROCHLORIDE (UNII: 64O047KTOA) (CETIRIZINE - UNII:YO7261ME24) CETIRIZINE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color WHITE Score no score Shape ROUND Size 6mm Flavor Imprint Code M;C35 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0378-3635-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/27/2007 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076677 12/27/2007 CETIRIZINE HYDROCHLORIDE
cetirizine hydrochloride tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0378-3637 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE HYDROCHLORIDE (UNII: 64O047KTOA) (CETIRIZINE - UNII:YO7261ME24) CETIRIZINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color WHITE Score no score Shape ROUND Size 8mm Flavor Imprint Code M;C37 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0378-3637-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/27/2007 2 NDC:0378-3637-05 500 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/27/2007 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076677 12/27/2007 Labeler - Mylan Pharmaceuticals Inc. (059295980)