Label: NEUTRARINSE 2% NEUTRAL SODIUM FLORIDE ORAL RINSE WHITE GRAPE- sodium fluoride rinse
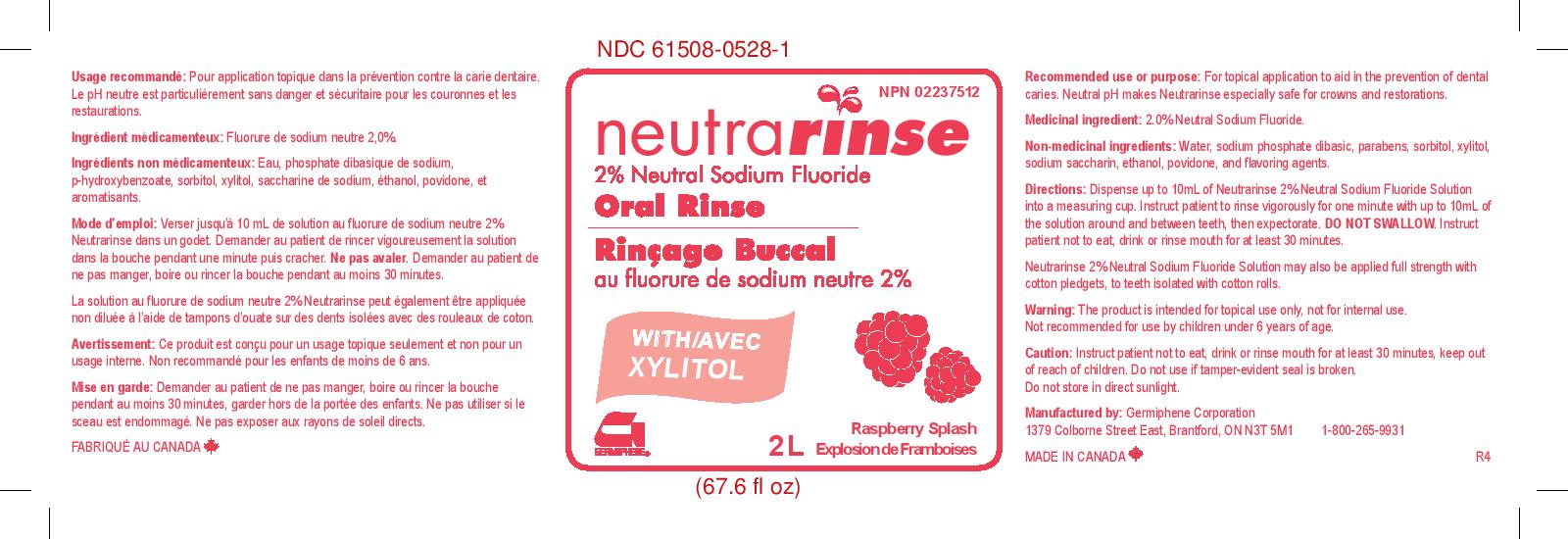
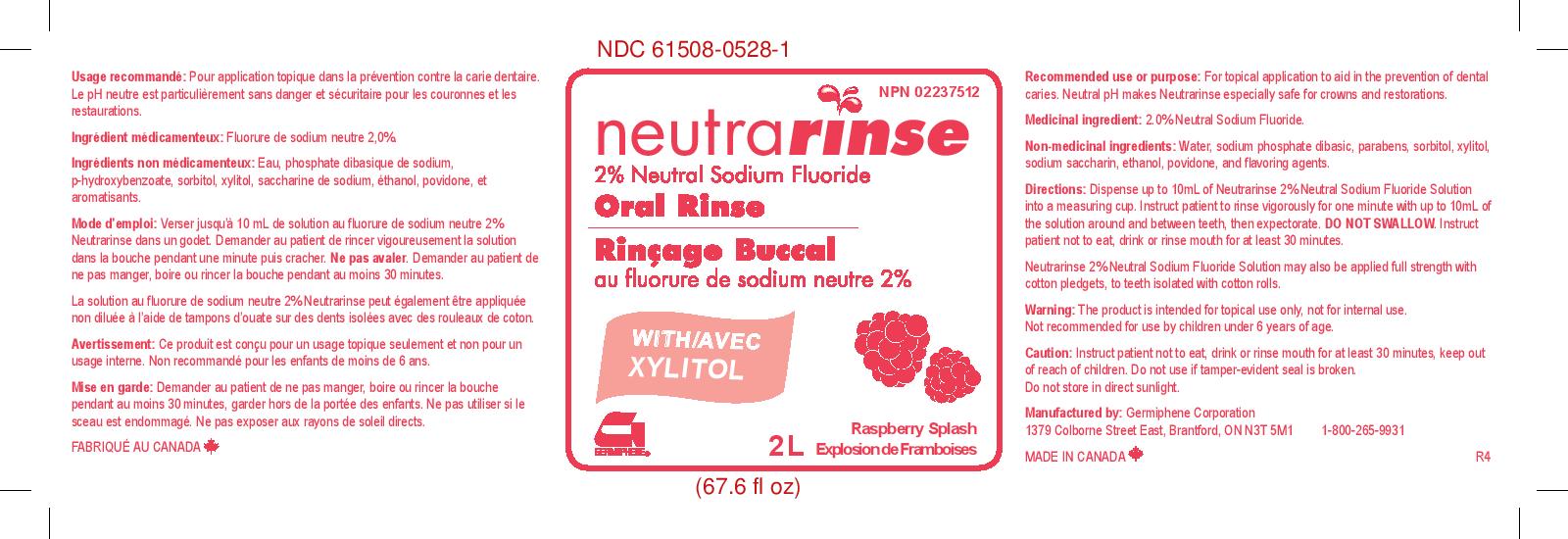
NEUTRARINSE 2% NEUTRAL SODIUM FLORIDE ORAL RINSE RASPBERRY SPLASH- sodium fluoride rinse
NEUTRARINSE 2% NEUTRAL SODIUM FLORIDE ORAL RINSE REFRESHMINT- sodium fluoride rinse
-
Contains inactivated NDC Code(s)
NDC Code(s): 61508-0526-1, 61508-0527-1, 61508-0528-1 - Packager: Germiphene Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated February 3, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Recommended use:
- PURPOSE
- Medicinal ingredient:
- Non-medicinal ingredients:
-
Directions:
Dispense up to 10 mL of Neutrarinse 2% Neutral Sodium Fluoride Solution into a measuring cup. Instruct patient to rinse vigorously for one minute with up to 10 mL of the solution arond and between teeth, then expectorate. DO NOT SWALLOW. Instruct patient not to eat, drink, or rinse mouth for at least 30 minutes.
Neutrarinse 2% Neutral Sodium Fluoride Solution may also be applied full strength with cotton pledgets, to teeth isolated with cotton rolls.
- Warning:
- KEEP OUT OF REACH OF CHILDREN
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NEUTRARINSE 2% NEUTRAL SODIUM FLORIDE ORAL RINSE WHITE GRAPE
sodium fluoride rinseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61508-0527 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 2 g in 100 g Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) 1 g in 100 g METHYLPARABEN (UNII: A2I8C7HI9T) 0.052 g in 100 g POVIDONE (UNII: FZ989GH94E) 0.3 g in 100 g SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) 0.043 g in 100 g SORBITOL (UNII: 506T60A25R) 15 g in 100 g WATER (UNII: 059QF0KO0R) 76.4 g in 100 g XYLITOL (UNII: VCQ006KQ1E) 5 g in 100 g SACCHARIN SODIUM (UNII: SB8ZUX40TY) 0.01 g in 100 g PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.01 g in 100 g Product Characteristics Color Score Shape Size Flavor GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61508-0527-1 2000 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/06/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 02/06/2017 NEUTRARINSE 2% NEUTRAL SODIUM FLORIDE ORAL RINSE RASPBERRY SPLASH
sodium fluoride rinseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61508-0528 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 2 g in 100 g Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) 1 g in 100 g METHYLPARABEN (UNII: A2I8C7HI9T) 0.052 g in 100 g POVIDONE (UNII: FZ989GH94E) 0.3 g in 100 g SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) 0.043 g in 100 g SORBITOL (UNII: 506T60A25R) 15 g in 100 g WATER (UNII: 059QF0KO0R) 76.4 g in 100 g XYLITOL (UNII: VCQ006KQ1E) 5 g in 100 g SACCHARIN SODIUM (UNII: SB8ZUX40TY) 0.01 g in 100 g PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.01 g in 100 g Product Characteristics Color Score Shape Size Flavor BERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61508-0528-1 2000 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/06/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 02/06/2017 NEUTRARINSE 2% NEUTRAL SODIUM FLORIDE ORAL RINSE REFRESHMINT
sodium fluoride rinseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61508-0526 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 2 g in 100 g Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) 1 g in 100 g METHYLPARABEN (UNII: A2I8C7HI9T) 0.052 g in 100 g POVIDONE (UNII: FZ989GH94E) 0.3 g in 100 g SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) 0.043 g in 100 g SORBITOL (UNII: 506T60A25R) 15 g in 100 g WATER (UNII: 059QF0KO0R) 76.4 g in 100 g XYLITOL (UNII: VCQ006KQ1E) 5 g in 100 g SACCHARIN SODIUM (UNII: SB8ZUX40TY) 0.01 g in 100 g PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.01 g in 100 g Product Characteristics Color Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61508-0526-1 2000 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/06/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 02/06/2017 Labeler - Germiphene Corporation (206412512) Establishment Name Address ID/FEI Business Operations Germiphene Corporation 206412512 manufacture(61508-0526, 61508-0527, 61508-0528)