Label: THYROSAFE- potassium iodide tablet
- NDC Code(s): 50633-910-20
- Packager: BTG International Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 22, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
Take potassium iodide (KI) only when public officials tell you. In a nuclear radiation emergency, radioactive iodine could be released into the air. KI protects only the thyroid gland from uptake of radioactive iodine. Therefore, KI should be used along with other emergency measures that will be recommended to you by public officials. If you are told to take this medicine, take it 1 time every 24 hours. Do not take it more often. More KI will not help you. Too much KI may increase the chances of side effects. Do not take this medicine if you know you are allergic to iodine (see SIDE EFFECTS below).
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
DOSAGE & ADMINISTRATION
Dose:
Adults over 18 years 2 tablets (whole or crushed) every day (130 mg) Children over 12 years to 18 years who weigh at least 150 pounds 2 tablets (whole or crushed) every day (130 mg) Children over 12 years to 18 years who weigh less than 150 pounds 1 tablet (whole or crushed) or 8 teaspoons every day (65 mg) Children over 3 years to 12 years 1 tablet (whole or crushed) or 8 teaspoons every day (65 mg) Children over 1 month to 3 years 4 teaspoons every day (32.5 mg) Babies at birth to 1 month 2 teaspoons every day (16.25 mg) Tablets can be crushed and mixed in many liquids. To take the tablet in liquid solution, use dosing directions under Making a Potassium Iodide Liquid Mixture.
Take KI every day (every 24 hours) as directed by public officials. Do not take more than 1 dose in 24 hours. More will not help you. Too much medicine may increase the chances of side effects.
Making a Potassium Iodide Liquid Mixture:
- Put one 65 mg KI tablet into a small bowl and grind it into a fine powder using the back of a metal teaspoon against the inside of the bowl. The powder should not have any large pieces.
- Add 4 teaspoons of water to the crushed KI powder in the bowl and mix until the KI powder is dissolved in the water.
- Take the KI water mixture solution made in step 2 and mix it with 4 teaspoons of low fat white or chocolate milk, orange juice, flat soda, raspberry syrup, or infant formula.
- The KI liquid mixture will keep for up to 7 days in the refrigerator. It is recommended that the KI liquid mixtures be prepared weekly. Throw away unused portions.
The amount of KI (65 mg tablet) in the drink when mixed as described above is 8.125 mg per teaspoon. The number of teaspoons of the drink to give your child depends on your child’s age as described in the following table:
Child’s Age Give your child this amount in teaspoons Over 12 to 18 years old who weigh less than 150 pounds 8 teaspoons will give you a 65 mg dose Over 3 to 12 years old 8 teaspoons will give you a 65 mg dose Over 1 month to 3 years old 4 teaspoons will give you a 32.5 mg dose Birth to 1 month 2 teaspoons will give you a 16.25 mg dose Note: This is the amount to give your child for one single dose in teaspoons (not tablespoons). You should give your child one dose each day as recommended by the public officials.
Pregnant or breastfeeding women or babies under 1 month of age: Take as directed above and call a doctor as soon as possible. Repeat dosing should be avoided. It is recommended that thyroid function be checked in babies less than 1 month of age that take KI. Women who are pregnant or breastfeeding should also be checked by a doctor if repeat dosing is necessary. Although these precautions should be taken, the benefits of short-term use of KI to block uptake of radioactive iodine by the thyroid gland far exceed its chances of side effects.
Patients with thyroid disease: If you have both a nodular thyroid condition such as multinodular goiter with heart disease, you should not take KI. Patients with other thyroid conditions may take KI as directed above, but call a doctor if you need to take KI for more than a few days.
-
WARNINGS
WARNING
People who are allergic to iodine, have dermatitis herpetiformis or hypocomplementemic vasculitis, or have nodular thyroid disease with heart disease should not take KI. Keep out of the reach of children. In case of an allergic reaction (difficulty breathing, speaking or swallowing; wheezing; shortness of breath or swelling of the mouth or throat), call 911 or get medical care right away. In case of overdose, get medical help or call a Poison Control Center right away.
-
SPL UNCLASSIFIED SECTION
HOW POTASSIUM IODIDE WORKS
Certain forms of iodine help your thyroid gland work right. Most people get the iodine they need from foods like iodized salt or fish. The thyroid can "store" or hold only a certain amount of iodine.
In a nuclear radiation emergency, radioactive iodine may be released in the air. This material may be breathed or swallowed. It may enter the thyroid gland and damage it. The damage would probably not show itself for years. Children are most likely to have thyroid damage.
If you take KI, it will block or reduce the chances that radioactive iodine will enter your thyroid gland.
- SPL UNCLASSIFIED SECTION
-
SPL UNCLASSIFIED SECTION
HOW AND WHEN TO TAKE POTASSIUM IODIDE
KI should be taken as soon as possible after public officials tell you. If you are told to repeat the dose, you should take the second dose 24 hours after the first dose. Do not take it sooner. More KI will not help you because the thyroid can "hold" only certain amounts of iodine. Taking more than 1 dose per day will increase the chances of side effects. The public officials will tell you how many days to take KI. You should take KI until the chances of major exposure to radioactive iodine by breathing or swallowing stops.
-
SPL UNCLASSIFIED SECTION
SIDE EFFECTS
Short-term use of KI at the recommended dose is safe. You should not take this drug for longer than you are told.
Possible side effects include: swelling of the salivary glands, nausea, vomiting, diarrhea, stomach ache, fever, headache, metallic taste, and allergic reactions. Allergic reactions can include
- skin rashes such as hives
- swelling of various parts of the body such as the face, lips, tongue, throat, hands or feet
- fever with joint pain
- trouble breathing, speaking or swallowing
- wheezing or shortness of breath
Get medical attention right away if you have trouble breathing, speaking or swallowing; wheezing; shortness of breath; or swelling of the mouth, tongue or throat.
Taking iodide, in rare cases, may cause overactivity of the thyroid gland, underactivity of the thyroid gland, or enlargement of the thyroid gland (goiter). Symptoms of an overactive thyroid gland may include an irregular heart beat and chest pain. Patients with thyroid disease are more likely to get these side effects. Babies under 1 month of age are more likely to get an underactive thyroid gland (hypothyroidism).
-
SPL UNCLASSIFIED SECTION
WHAT TO DO IF SIDE EFFECTS OCCUR
Stop taking KI and call a doctor if you have one or more of the following symptoms:
- swelling of the face, hands or feet
- fever and joint pain
- skin rash
Stop taking KI and get medical help right away if you have one or more of the following symptoms:
- trouble breathing, speaking or swallowing
- shortness of breath or wheezing
- swelling of the lips, tongue or throat
- irregular heart beat or chest pain
-
SPL UNCLASSIFIED SECTION
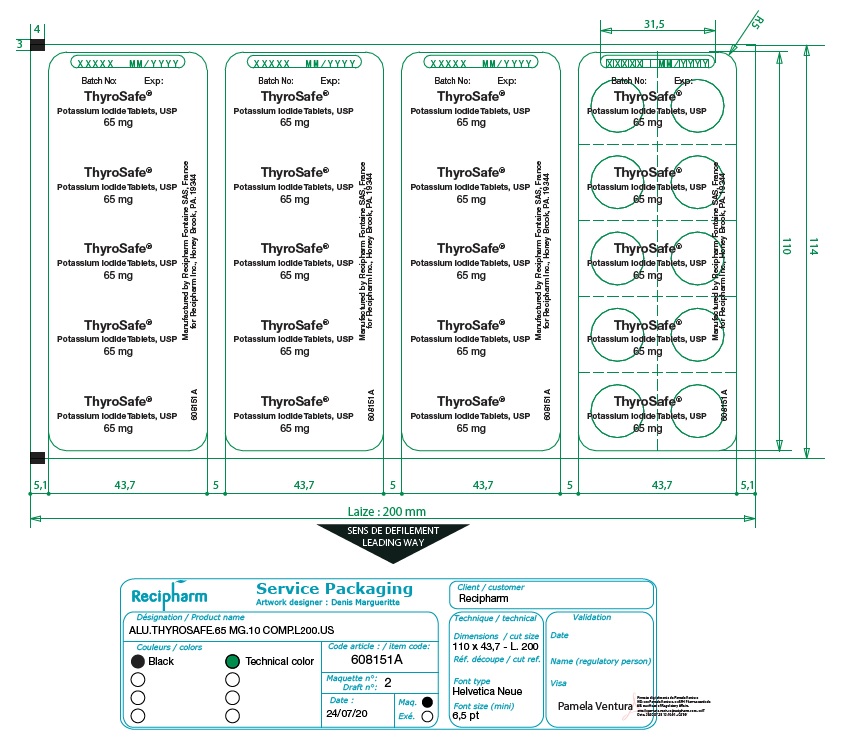
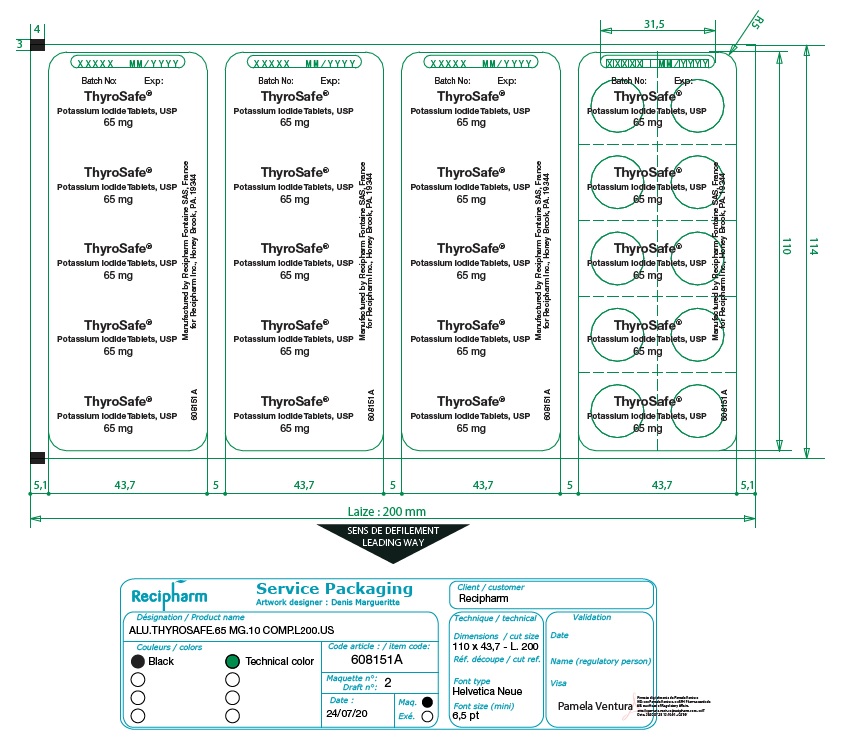
HOW SUPPLIED
ThyroSafe® (potassium iodide, USP) tablets. Packages of 10 and 20 tablets. Each white, round, cross-scored tablet contains 65 mg potassium iodide. Store at 20-25° C (68-77° F). Keep dry and foil intact.
Manufactured by Astrea Fontaine SAS,
21121 Fontaine les Dijon, France
for BTG International Inc., West Conshohocken, PA 19428 USA
1-866-849-7672.
www.thyrosafe.com.
Revised 02/2022
- SPL UNCLASSIFIED SECTION
- INACTIVE INGREDIENT
-
KEEP OUT OF REACH OF CHILDREN
Carton Label - Principal Display Panel
NDC-50633-910-20
ThyroSafe®
Potassium Iodide Tablets, USP, 65 mg
Thyroid blocking in a radiation emergency only
20 tablets, 65 milligrams eachCarton Label – Back panel
Potassium Iodide (KI) Tablets USP, 65 mg
Drug Facts Active Ingredient (in each tablet)
Potassium Iodide 65 mgPurpose
Thyroid blockingUse helps prevent radioactive iodine from getting into the thyroid gland during a nuclear radiation emergency. Use along with other emergency measures recommended by public officials. Warnings
Allergy alert: Iodine may cause an allergic reaction with 1 or more of the following symptoms:- shortness of breath or wheezing
- swelling
- skin rash
- trouble breathing, speaking or swallowing
- fever and joint pain
Do not use if you have - ever had an allergic reaction to iodine
- nodular thyroid disease with heart disease
- hypocomplementemic vasculitis
- dermatitis herpetiformis
Stop use and ask a doctor if you have if you have - allergic reaction. Get medical help right away if you have trouble breathing, speaking or swallowing; shortness of breath; wheezing; swelling of the mouth, tongue or throat; or rash.
- irregular heart or chest pain. Get medical help right away.
- swelling of the hands or feet, fever, or joint pain.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. Directions - use as directed by public officials in the event of a nuclear radiation emergency.
- do not take more than 1 dose in 24 hours.
- tablets can be whole or crushed and mixed in milk, baby formula, water, orange juice, flat soda like cola, or raspberry syrup. The liquid mixture should be given to infants, young children, and others who cannot swallow tablets; see consumer package insert on how to make a liquid mixture.
adults over 18 years 2 tablets (whole or crushed) daily (130 mg) children over 12 years to 18 years who weigh at least 150 pounds 2 tablets (whole or crushed) daily (130 mg) children over 12 years to 18 years who weigh less than 150 pounds 1 tablet (whole or crushed) daily (65 mg) children over 3 years to 12 years 1 tablet (whole or crushed) daily (65 mg) children over 1 month to 3 years 1/2 tablet (crushed) daily (32.5 mg) babies at birth to 1 month 16.25 mg daily as directed in consumer package insert If pregnant, breastfeeding, have a baby up to 1 month of age, or have thyroid disease (except nodular thyroid disease with heart disease), take as directed above and contact a doctor as soon as possible - Principal Display Panel - 65 mg Carton Label
- Principal Display Panel - Pack Label
-
INGREDIENTS AND APPEARANCE
THYROSAFE
potassium iodide tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50633-910 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Potassium Iodide (UNII: 1C4QK22F9J) (Iodide Ion - UNII:09G4I6V86Q) Potassium Iodide 65 mg Inactive Ingredients Ingredient Name Strength Lactose, Unspecified form (UNII: J2B2A4N98G) Microcrystalline Cellulose (UNII: OP1R32D61U) Magnesium Stearate (UNII: 70097M6I30) Product Characteristics Color white (white) Score 4 pieces Shape ROUND (ROUND) Size 9mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50633-910-20 160 in 1 CARTON 09/30/2002 1 20 in 1 BOX 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076350 09/30/2002 Labeler - BTG International Inc. (617382395) Establishment Name Address ID/FEI Business Operations Astrea Fontaine 260428962 MANUFACTURE(50633-910)