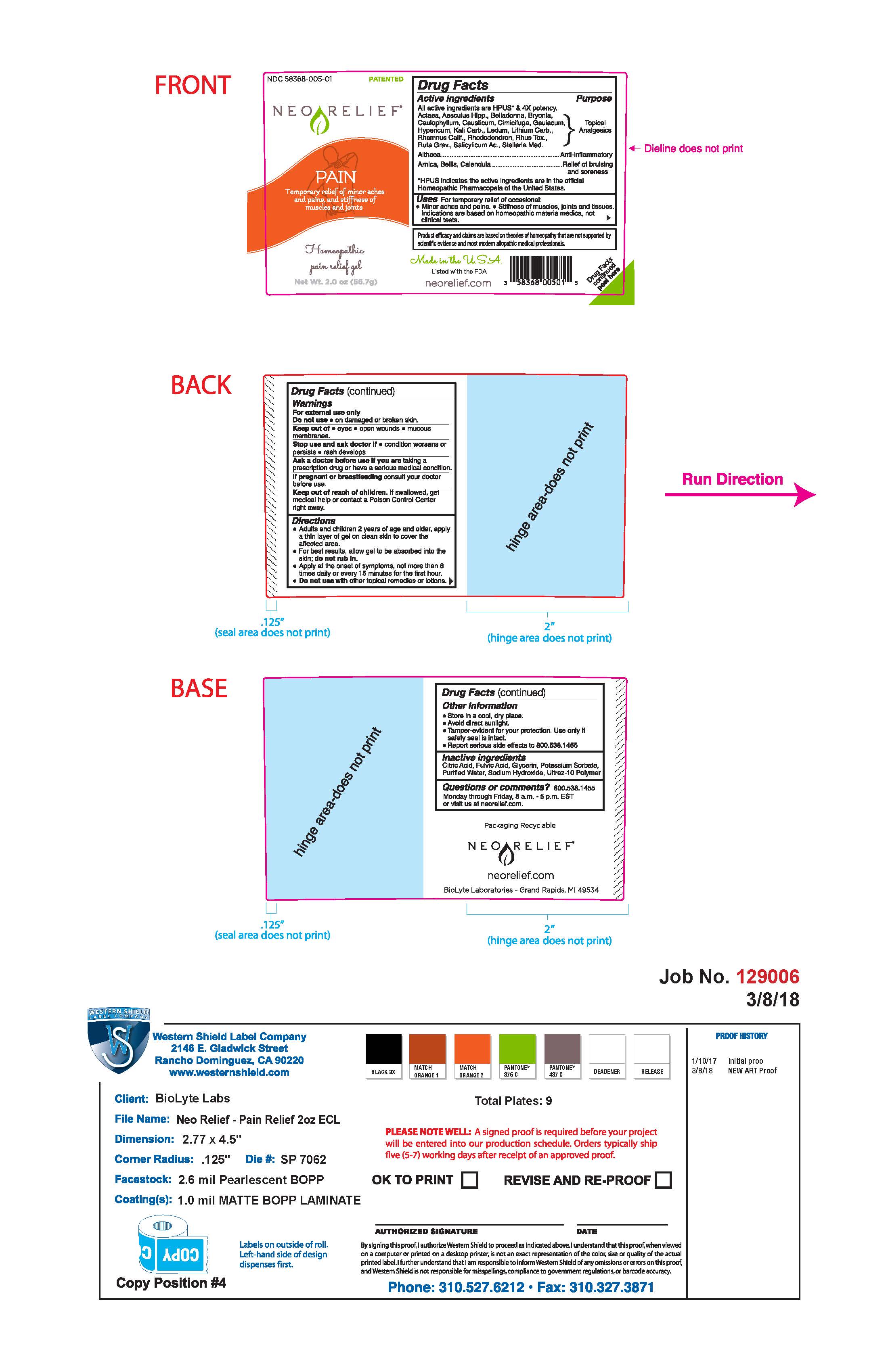
Label: NEORELIEF FOR PAIN- topical gel for pain gel
- NDC Code(s): 58368-005-01
- Packager: BioLyte Laboratories, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Drug Facts Active Ingredients
All active ingredients are HPUS* and 4X potency
Actaea, Aesculus Hipp., Belladonna, Bryonia., Caulophyllum, Causticum, Cimicifuga, Gauiacum, Hypericum, Kali Carb., Ledum, Lithium Carb., Rhamnus Calif., Rhododendron, Rhus. Tox., Ruta Grav., Salicylicum Ac., Setllaria Med.
Althaea
Arnica, Bellis, Calendula
- Purpose
- Uses
- Warnings
- Warnings
- Warnings
- Warnings
- Warnings
-
Directions
- Adults and children 2 years of age and older, apply a thin layer of gel on clean skin to cover the affected area.
- For best results, allow gel to be absorbed into the skin; do not rub in.
- Apply at the onset of symptoms, not more than 6 times daily or every 15 minutes for the first hour.
- Do not use with other topical remedies or lotions.
- Other Information
- Inactive Ingredients
- Questions or comments?
-
Additional Label Content
Required FTC Disclosure: Product efficacy and claims are based on theories of homeopathy that are not supported by scientific evidence and most modern allopathic medical professionals.
Made in the USA
Listed with the FDA
(UPC code: 3 58368 00501 5)
Drug Facts continued Peel Here
Packaging Recyclable
neorelief.com
BioLyte laboratories- Grand Rapids, MI 49534
- NEORELIEF for PAIN 56.7g Bottle
-
INGREDIENTS AND APPEARANCE
NEORELIEF FOR PAIN
topical gel for pain gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58368-005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BELLIS PERENNIS (UNII: 2HU33I03UY) (BELLIS PERENNIS - UNII:2HU33I03UY) BELLIS PERENNIS 4 [hp_X] in 1 g BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 4 [hp_X] in 1 g CALENDULA OFFICINALIS FLOWERING TOP (UNII: 18E7415PXQ) (CALENDULA OFFICINALIS FLOWERING TOP - UNII:18E7415PXQ) CALENDULA OFFICINALIS FLOWERING TOP 4 [hp_X] in 1 g CAULOPHYLLUM THALICTROIDES ROOT (UNII: JTJ6HH6YEH) (CAULOPHYLLUM THALICTROIDES ROOT - UNII:JTJ6HH6YEH) CAULOPHYLLUM THALICTROIDES ROOT 4 [hp_X] in 1 g CAUSTICUM (UNII: DD5FO1WKFU) (CAUSTICUM - UNII:DD5FO1WKFU) CAUSTICUM 4 [hp_X] in 1 g GUAIACUM OFFICINALE RESIN (UNII: N0K2Z502R6) (GUAIACUM OFFICINALE RESIN - UNII:N0K2Z502R6) GUAIACUM OFFICINALE RESIN 4 [hp_X] in 1 g HYPERICUM PERFORATUM (UNII: XK4IUX8MNB) (HYPERICUM PERFORATUM - UNII:XK4IUX8MNB) HYPERICUM PERFORATUM 4 [hp_X] in 1 g LITHIUM CARBONATE (UNII: 2BMD2GNA4V) (LITHIUM CATION - UNII:8H8Z5UER66) LITHIUM CARBONATE 4 [hp_X] in 1 g FRANGULA CALIFORNICA BARK (UNII: 1LZ13MQR0S) (FRANGULA CALIFORNICA BARK - UNII:1LZ13MQR0S) FRANGULA CALIFORNICA BARK 4 [hp_X] in 1 g RHODODENDRON AUREUM LEAF (UNII: IV92NQJ73U) (RHODODENDRON AUREUM LEAF - UNII:IV92NQJ73U) RHODODENDRON AUREUM LEAF 4 [hp_X] in 1 g TOXICODENDRON PUBESCENS LEAF (UNII: 6IO182RP7A) (TOXICODENDRON PUBESCENS LEAF - UNII:6IO182RP7A) TOXICODENDRON PUBESCENS LEAF 4 [hp_X] in 1 g RUTA GRAVEOLENS FLOWERING TOP (UNII: N94C2U587S) (RUTA GRAVEOLENS FLOWERING TOP - UNII:N94C2U587S) RUTA GRAVEOLENS FLOWERING TOP 4 [hp_X] in 1 g HORSE CHESTNUT (UNII: 3C18L6RJAZ) (HORSE CHESTNUT - UNII:3C18L6RJAZ) HORSE CHESTNUT 4 [hp_X] in 1 g SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 4 [hp_X] in 1 g BLACK COHOSH (UNII: K73E24S6X9) (BLACK COHOSH - UNII:K73E24S6X9) BLACK COHOSH 4 [hp_X] in 1 g STELLARIA MEDIA (UNII: 2H03479QVR) (STELLARIA MEDIA - UNII:2H03479QVR) STELLARIA MEDIA 4 [hp_X] in 1 g ACTAEA SPICATA ROOT (UNII: 3FU86L9OS0) (ACTAEA SPICATA ROOT - UNII:3FU86L9OS0) ACTAEA SPICATA ROOT 4 [hp_X] in 1 g ALTHAEA OFFICINALIS ROOT (UNII: TRW2FUF47H) (ALTHAEA OFFICINALIS ROOT - UNII:TRW2FUF47H) ALTHAEA OFFICINALIS ROOT 4 [hp_X] in 1 g ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 4 [hp_X] in 1 g ATROPA BELLADONNA ROOT (UNII: 6MW97Q6E8M) (ATROPA BELLADONNA ROOT - UNII:6MW97Q6E8M) ATROPA BELLADONNA ROOT 4 [hp_X] in 1 g POTASSIUM CARBONATE (UNII: BQN1B9B9HA) (CARBONATE ION - UNII:7UJQ5OPE7D) POTASSIUM CARBONATE 4 [hp_X] in 1 g LEDUM PALUSTRE TWIG (UNII: 877L01IZ0P) (LEDUM PALUSTRE TWIG - UNII:877L01IZ0P) LEDUM PALUSTRE TWIG 4 [hp_X] in 1 g Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) FULVIC ACID (UNII: XII14C5FXV) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58368-005-01 1 in 1 CARTON 02/01/2017 1 56.7 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/01/2017 Labeler - BioLyte Laboratories, LLC (015560564) Establishment Name Address ID/FEI Business Operations BioLyte Laboratories, LLC 015560564 manufacture(58368-005)