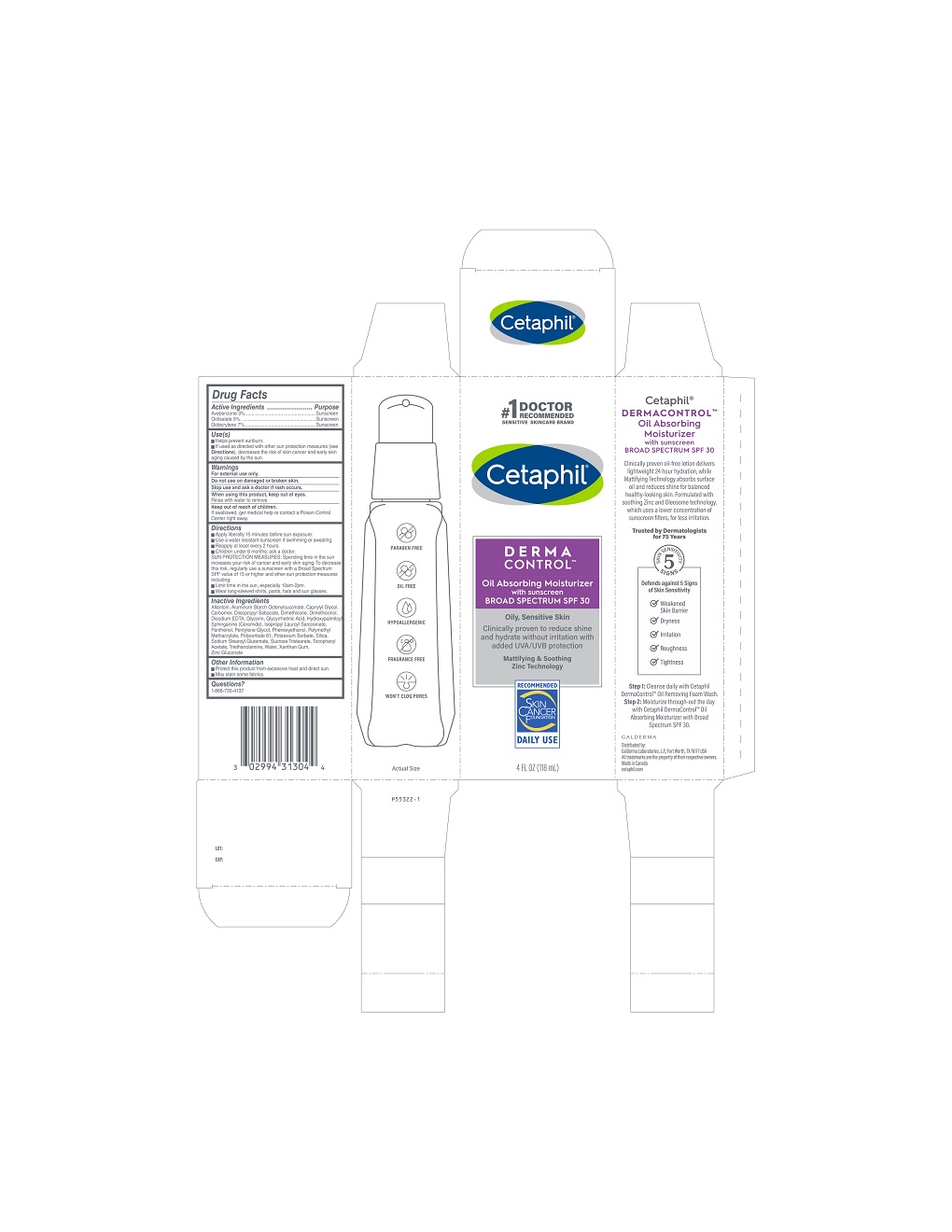
Label: CETAPHIL OIL ABSORBING SPF 30- octocrylene, octisalate, avobenzone lotion
- NDC Code(s): 0299-4123-00, 0299-4123-05
- Packager: Galderma Laboratories, L.P.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 1, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Use(s)
- Warnings
- Keep out of reach of children
-
Directions
• Apply liberally 15 minutes before sun exposure.
• Use a water resistant sunscreen if swimming or sweating.
• Reapply at least every 2 hours.
• Children under 6 months: Ask a doctor.SUN PROTECTION MEASURES: Spending time in the sun increases your risk of cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
• Limit time in the sun, especially 10am - 2pm.
• Wear long-sleeved shirts, pants, hats, and sunglasses.
-
Inactive Ingredients
Allantoin, Aluminum Starch Octenylsuccinate, Caprylyl Glycol, Carbomer, Diisopropyl Sebacate, Dimethicone, Dimethiconol, Disodium EDTA, Glycerin, Glycyrrhetinic Acid, Hydroxypalmitoyl Sphinganine (Ceramide), Isopropyl Lauroyl Sarcosinate, Panthenol, Pentylene Glycol, Phenoxyethanol, Polymethyl Methacrylate, Polysorbate 61, Potassium Sorbate, Silica, Sodium Stearoyl Glutamate, Sucrose Tristearate, Tocopheryl Acetate, Triethanolamine, Water, Xanthan Gum, Zinc Gluconate
- Other Information
- Questions?
-
PRINCIPLE DISPLAY PANEL - 4oz carton
#1 Doctor Recommended
Sensitive Skincare Brand
Cetaphil®
DERMACONTROLTM
Oil Absorbing Moisturizer
with sunscreen
BROAD SPECTRUM SPF 30
Oily, Sensitive Skin
Clinically proven to reduce shine
and hydrate without irritation with
added UVA/UVB protection
Mattifying and Soothing
Zinc Technology
Skin Cancer Foundation logo
4 FL OZ (118 mL)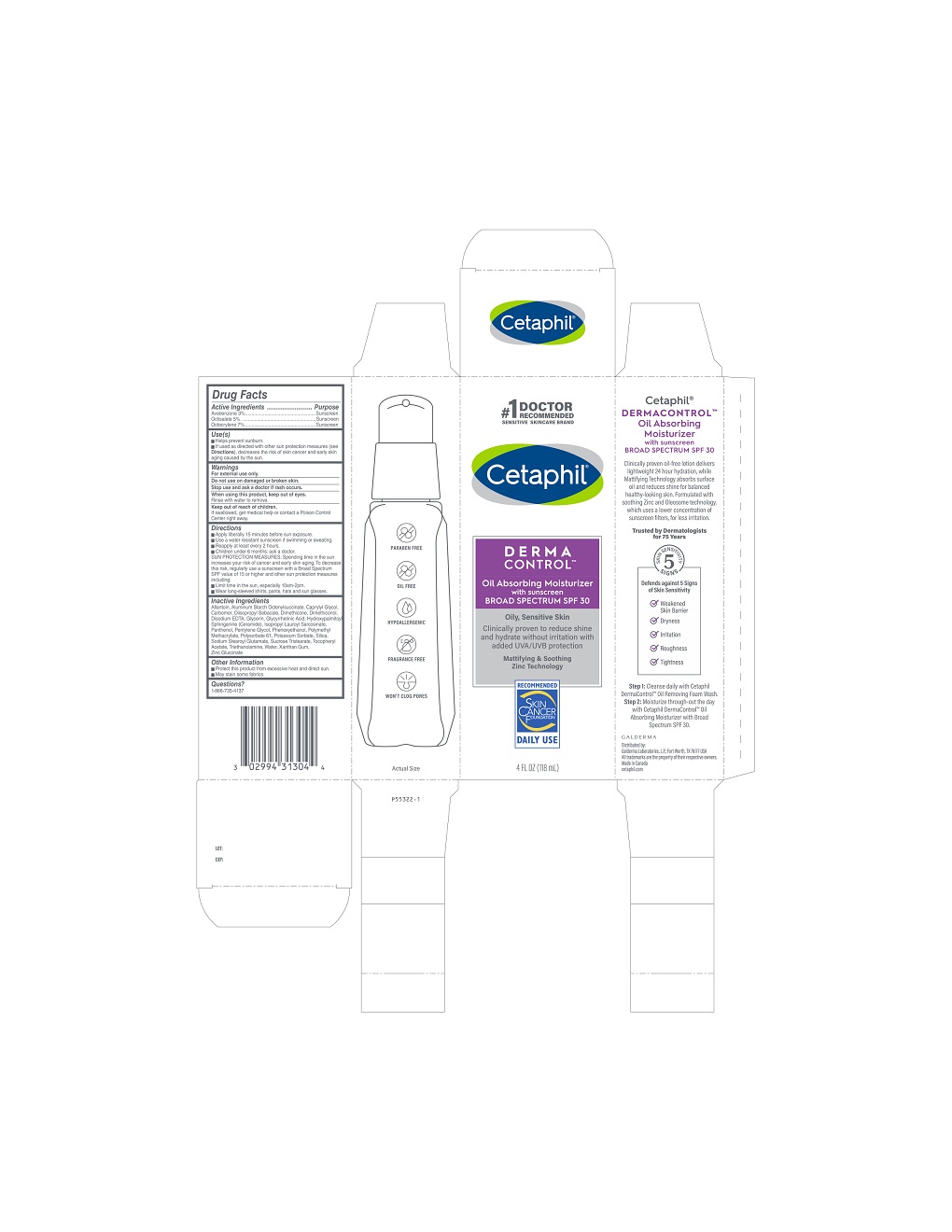
-
INGREDIENTS AND APPEARANCE
CETAPHIL OIL ABSORBING SPF 30
octocrylene, octisalate, avobenzone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0299-4123 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octocrylene (UNII: 5A68WGF6WM) (Octocrylene - UNII:5A68WGF6WM) Octocrylene 7 g in 100 mL Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 5 g in 100 mL Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 3 g in 100 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Dimethicone (UNII: 92RU3N3Y1O) Diisopropyl Sebacate (UNII: J8T3X564IH) Silicon Dioxide (UNII: ETJ7Z6XBU4) Aluminum Starch Octenylsuccinate (UNII: I9PJ0O6294) Pentylene Glycol (UNII: 50C1307PZG) Sodium Stearoyl Glutamate (UNII: 65A9F4P024) Phenoxyethanol (UNII: HIE492ZZ3T) Caprylyl Glycol (UNII: 00YIU5438U) Enoxolone (UNII: P540XA09DR) Panthenol (UNII: WV9CM0O67Z) Trolamine (UNII: 9O3K93S3TK) Allantoin (UNII: 344S277G0Z) Potassium Sorbate (UNII: 1VPU26JZZ4) Zinc Gluconate (UNII: U6WSN5SQ1Z) Xanthan Gum (UNII: TTV12P4NEE) Edetate Disodium (UNII: 7FLD91C86K) Dimethiconol (2000 Cst) (UNII: T74O12AN6Y) Hydroxypalmitoyl Sphinganine (UNII: NR33W2353T) Isopropyl Lauroyl Sarcosinate (UNII: LYR06W430J) Poly(Methyl Methacrylate; 450000 Mw) (UNII: Z47NNT4J11) Polysorbate 61 (UNII: X9E1MY2JQG) Sucrose Tristearate (UNII: 71I93STU5M) Carbomer Homopolymer Type A (Allyl Pentaerythritol Crosslinked) (UNII: F68VH75CJC) .Alpha.-Tocopherol (UNII: H4N855PNZ1) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0299-4123-00 1 in 1 CARTON 12/01/2011 09/01/2025 1 118 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 2 NDC:0299-4123-05 10 mL in 1 TUBE; Type 0: Not a Combination Product 12/01/2011 09/01/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 12/01/2011 09/01/2025 Labeler - Galderma Laboratories, L.P. (047350186) Establishment Name Address ID/FEI Business Operations G Production Inc. 251676961 manufacture(0299-4123)