Label: AL0038502 ALBA TROPICAL FRUIT KIDS SUNSCREEN SPF40- homosalate, octocrylene, ethylhexyl salicylate, avobenzone spray
-
Contains inactivated NDC Code(s)
NDC Code(s): 61995-2085-4 - Packager: The Hain Celestial Group, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 12, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
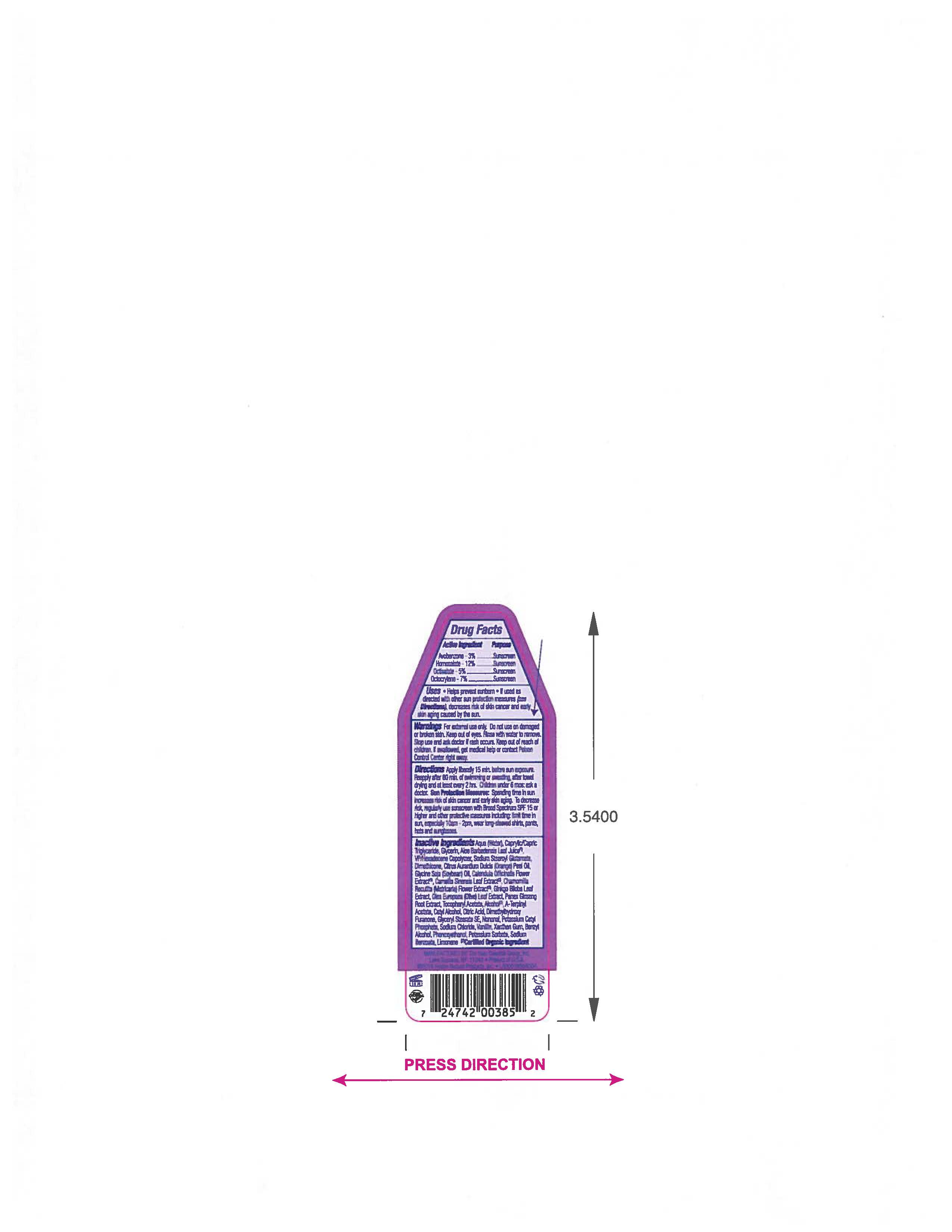
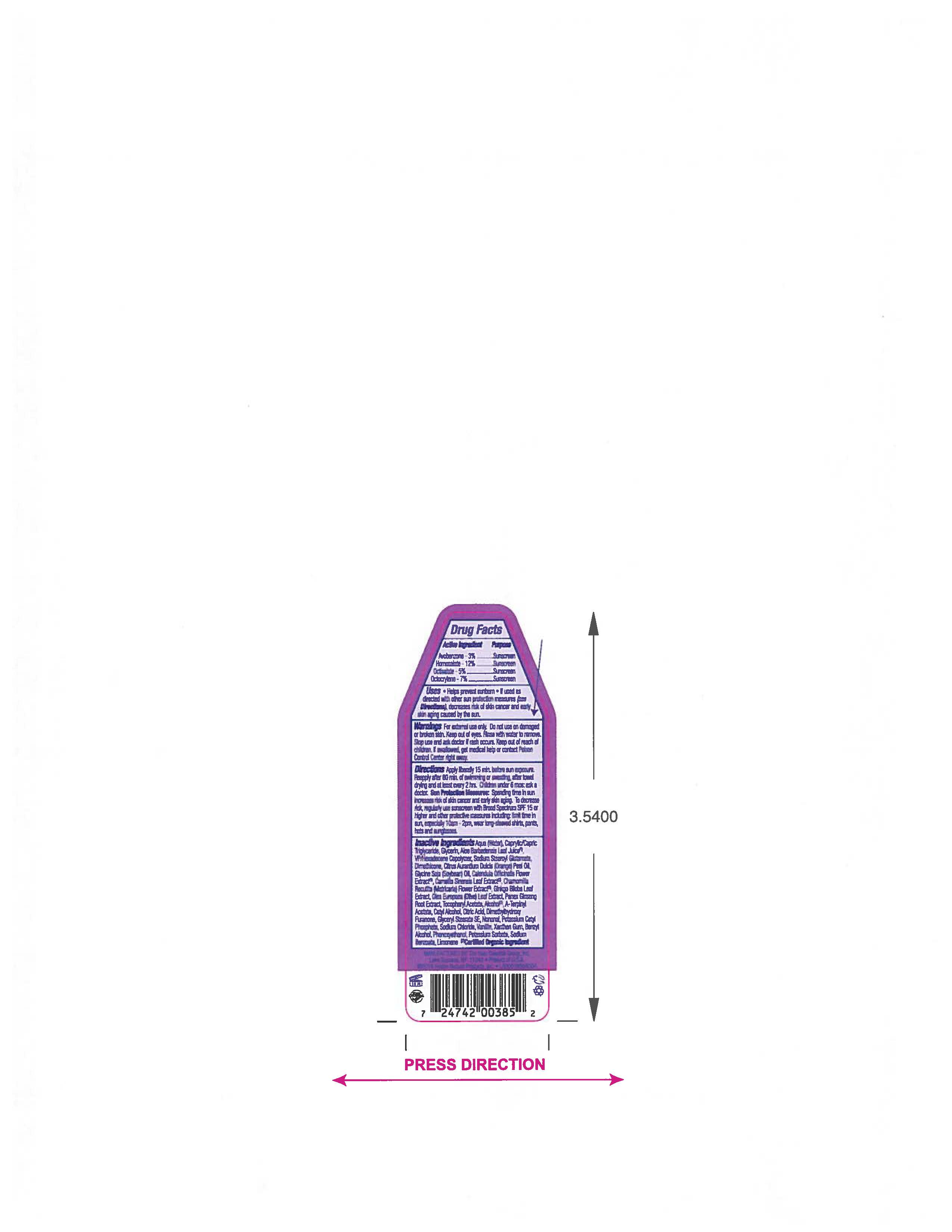
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Apply liberally 15 minutes before sun exposure. Reapply after 80 min of swimming or sweating,after towel drying and at lease every two hrs. Children under 6 mos: ask a doctor.
Skin protection measures:
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease a risk,regularly use a Sunscreen with Broad Spectrum SPF 15 or higher and other protective measures including: limit time in sun, especially 10am- 2pm, wear long- sleeved shirts,pants, hats and sunglasses.
-
INACTIVE INGREDIENT
Aqua (Water), Caprylic/Capric Triglyceride, Glycerin, Aloe Barbadensis Leaf Juice (1), VP/Hexadecene Copolymer, Sodium Stearoyl Glutamate, Dimethicone, Citrus Aurantium Dulcis (Orange) Peel Oil, Glycine Soja (Soybean) Oil, Calendula Officinalis Flower Extract (1), Camellia Sinensis Leaf Extract (1), Chamomilla Recuitita (Matricaria) Flower Extract (1), Gingko Biloba Root Extract, Olea Europaea (Olive) Leaf Extract, Panax Ginseng Root Extract (1), Tocopheryl Acetate, Alcohol (1), A-Terpinyl Acetate, Cetyl Alcohol, Citric Acid, Dimethylhydroxy Furanone, Glyceryl Stearate SE, Nonanal, Potassium Cetyl Phosphate, Sodium Chloride, Vanillin, Xanthan Gum, Benzyl Alcohol, Phenoxyethanol, Potassium Sorbate, Sodium Benzoate, Limonene.
(1) Certified Organic Ingredient - QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AL0038502 ALBA TROPICAL FRUIT KIDS SUNSCREEN SPF40
homosalate, octocrylene, ethylhexyl salicylate, avobenzone sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61995-2085 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 12 g in 100 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 g Inactive Ingredients Ingredient Name Strength VINYLPYRROLIDONE/HEXADECENE COPOLYMER (UNII: KFR5QEN0N9) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) DIMETHICONE (UNII: 92RU3N3Y1O) ALOE VERA LEAF (UNII: ZY81Z83H0X) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CHAMOMILE (UNII: FGL3685T2X) OLEA EUROPAEA LEAF (UNII: MJ95C3OH47) GINKGO (UNII: 19FUJ2C58T) ASIAN GINSENG (UNII: CUQ3A77YXI) CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) SODIUM CHLORIDE (UNII: 451W47IQ8X) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) XANTHAN GUM (UNII: TTV12P4NEE) ALCOHOL (UNII: 3K9958V90M) BENZYL ALCOHOL (UNII: LKG8494WBH) PHENOXYETHANOL (UNII: HIE492ZZ3T) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM BENZOATE (UNII: OJ245FE5EU) ORANGE OIL (UNII: AKN3KSD11B) SOYBEAN OIL (UNII: 241ATL177A) TERPINYL ACETATE (UNII: NIT9SZT3D7) DIMETHYLHYDROXY FURANONE (UNII: 20PI8YZP7A) NONANAL (UNII: 2L2WBY9K6T) VANILLIN (UNII: CHI530446X) LIMONENE, (+)- (UNII: GFD7C86Q1W) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61995-2085-4 118 g in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 09/13/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 09/13/2016 Labeler - The Hain Celestial Group, Inc. (117115556) Registrant - The Hain Celestial Group, Inc. (081512382) Establishment Name Address ID/FEI Business Operations The Hain Celestial Group, Inc. 081512382 manufacture(61995-2085)