Label: SYSTANE NIGHTTIME- mineral oil and white petrolatum ointment
- NDC Code(s): 0065-0509-35
- Packager: Alcon Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 15, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- Uses
- Warnings
- When using this product
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredient
- QUESTIONS
-
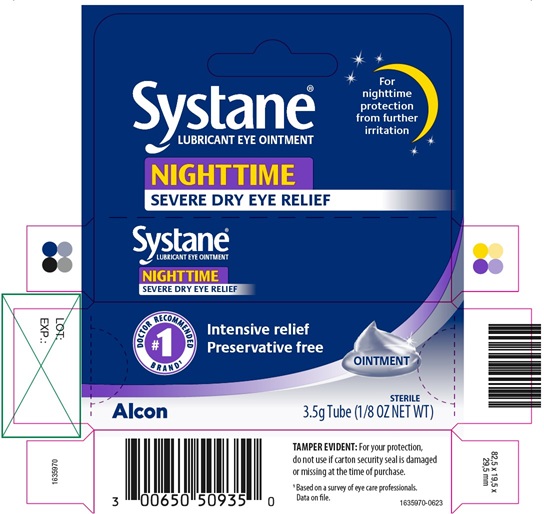
PRINCIPAL DISPLAY PANEL
Systane®
LUBRICANT EYE OINTMENT
NIGHTTIME
SEVERE DRY EYE RELIEF
For nighttime protection from further irritation
Systane®
LUBRICANT EYE OINTMENT
NIGHTTIME
SEVERE DRY EYE RELIEF
#1 DOCTOR RECOMMENDED BRAND1
Intensive relief
Preservative free
OINTMENT
Alcon
STERILE
3.5 g Tube (1/8 OZ NET WT)
TAMPER EVIDENT: For your protection,
do not use if carton security seal is damaged
or missing at the time of purchase.
1 Based on a survey of eye care professionals.
Data on file.
1635970-0623
LOT:
EXP.:
Systane®
LUBRICANT EYE OINTMENT
Alcon
Made in Belgium
ALCON LABORATORIES, INC.
Fort Worth, Texas 76134 USA

-
INGREDIENTS AND APPEARANCE
SYSTANE NIGHTTIME
mineral oil and white petrolatum ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0065-0509 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Mineral Oil (UNII: T5L8T28FGP) (Mineral Oil - UNII:T5L8T28FGP) Mineral Oil 30 mg in 1 g Petrolatum (UNII: 4T6H12BN9U) (Petrolatum - UNII:4T6H12BN9U) Petrolatum 940 mg in 1 g Inactive Ingredients Ingredient Name Strength Lanolin (UNII: 7EV65EAW6H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0065-0509-35 1 in 1 CARTON 06/26/2008 1 3.5 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 06/26/2008 Labeler - Alcon Laboratories, Inc. (008018525) Registrant - Alcon Laboratories, Inc. (008018525) Establishment Name Address ID/FEI Business Operations S.A. Alcon-Couvreur N.V. 370205429 manufacture(0065-0509)