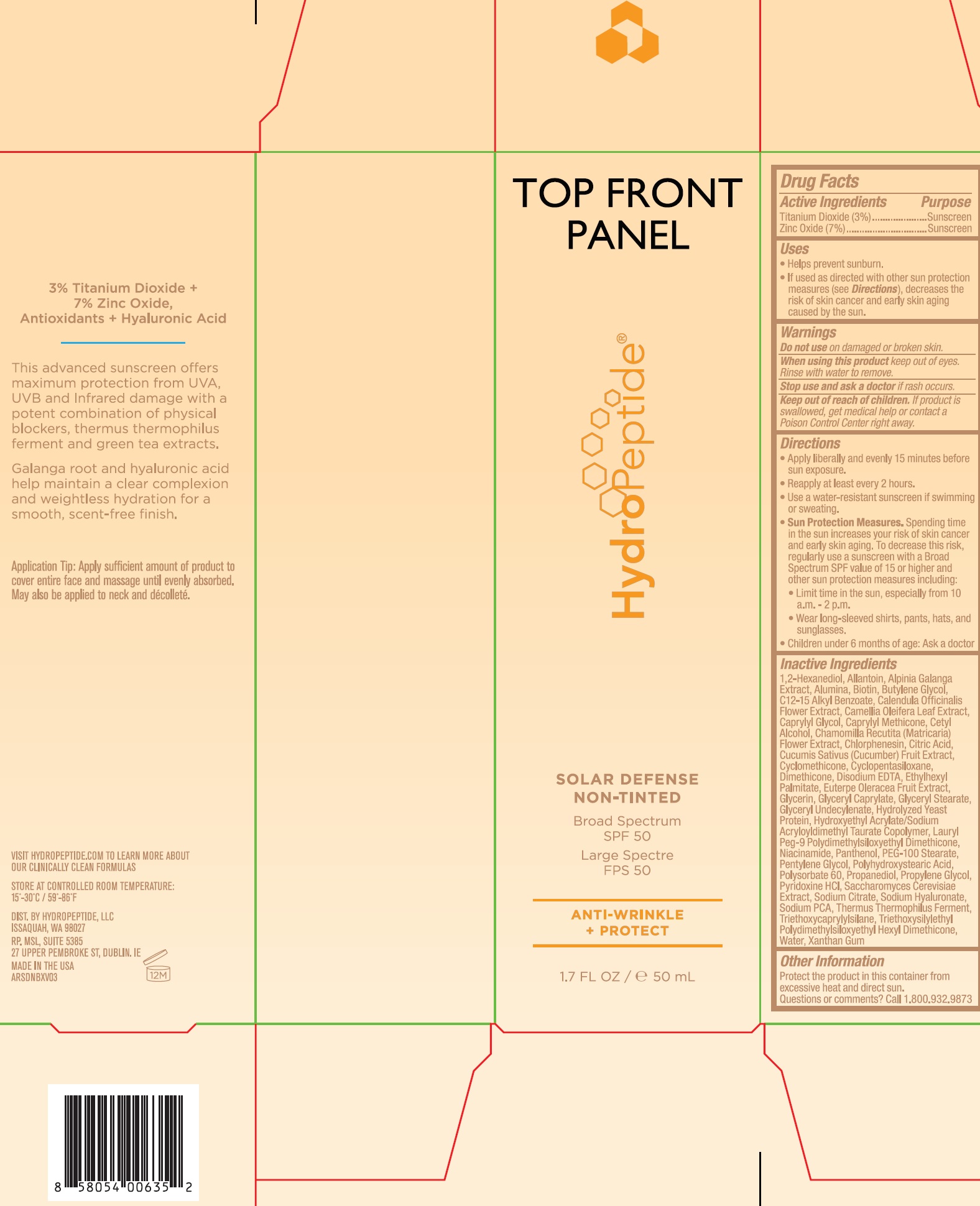
Label: SOLAR DEFENSE NON TINTED- titanium dioxide, zinc oxide cream
- NDC Code(s): 60934-003-01
- Packager: HydroPeptide LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 9, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Uses
- Warnings
-
Directions
- Apply liberally and evenly 15 minutes before sun exposure.
- Reapply at least every 2 hours.
- Use a water-resistant sunscreen if swimming or sweating.
- Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a suncreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: Sun Protection Measures.
- Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses.
- Children under 6 months of age: Ask a doctor
-
Inactive Ingredients
1,2-Hexanediol, Allantoin, Alpinia Galanga Extract, Alumina, Biotin, Butylene Glycol, C12-15 Alkly Benzoate, Calendula Officinalis Flower Extract, Camellia Oleifera Leaf Extract, Caprylyl Glycol, Caprylyl Methicone, Cetyl Alcohol, Chamomilla Recutita (Matricaria) Flower Extract, Chlorphenesin, Citric Acid Cucumis Sativus (Cucumber) Fruit Extract, Cyclomethicone, Cyclophentasiloxane, Dimethicone, Disodium EDTA, Ethylhexyl Palmitate, Euterpe Oleracea Fruit Extract, Glycerin, Glyceryl Caprylate, Glyceryl Stearate, Glyceryl Undecylenate, Hydrolyzed Yeast Protein, Hydroxyethyl Acylate/Sodium Acryloyldimethyl Taurate Copolymer, Lauryl Peg-9 Polydimethylsiloxyethyl Dimethicone, Niacinamide, Panthenol, PEG-100 Stearate, Pentylene Glycol, Polyhydroxystearic Acid, Polysorbate 60, Propanediol, Propylene Glycol, Pyridoxine HCl, Saccharomyces Cerevisiae Extract, Sodium Citrate, Sodium Hyaluronate, Sodium PCA, Thermus Thermophilus Ferment, Triethoxycaprylylsilane, Triethoxysilylethyl Polydimethylsiloxyethyl Hexyl Dimethicone, Water, Xanthan Gum
- Other Information
- Questions or comments?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
SOLAR DEFENSE NON TINTED
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:60934-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 30 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 70 mg in 1 mL Inactive Ingredients Ingredient Name Strength 1,2-HEXANEDIOL (UNII: TR046Y3K1G) ALLANTOIN (UNII: 344S277G0Z) ALUMINUM OXIDE (UNII: LMI26O6933) BIOTIN (UNII: 6SO6U10H04) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) CAMELLIA OLEIFERA LEAF (UNII: 5077EL0C60) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) CETYL ALCOHOL (UNII: 936JST6JCN) CHAMOMILE (UNII: FGL3685T2X) CHLORPHENESIN (UNII: I670DAL4SZ) CUCUMBER (UNII: YY7C30VXJT) CYCLOMETHICONE (UNII: NMQ347994Z) DIMETHICONE (UNII: 92RU3N3Y1O) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) ETHYLHEXYL PALMITATE (UNII: 2865993309) ACAI (UNII: 46AM2VJ0AW) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) GLYCERYL 1-UNDECYLENATE (UNII: B68LJT9544) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) NIACINAMIDE (UNII: 25X51I8RD4) PANTHENOL (UNII: WV9CM0O67Z) PEG-100 STEARATE (UNII: YD01N1999R) PENTYLENE GLYCOL (UNII: 50C1307PZG) POLYSORBATE 60 (UNII: CAL22UVI4M) PROPANEDIOL (UNII: 5965N8W85T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) SACCHAROMYCES CEREVISIAE (UNII: 978D8U419H) SODIUM CITRATE (UNII: 1Q73Q2JULR) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) THERMUS THERMOPHILUS LYSATE (UNII: 775R692494) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60934-003-01 1 in 1 BOX 01/11/2017 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/11/2017 Labeler - HydroPeptide LLC (006297465)