Label: M3 NATURALS ECZEMA CREAM- oatmeal cream
- NDC Code(s): 81985-186-01
- Packager: M3 Naturals LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
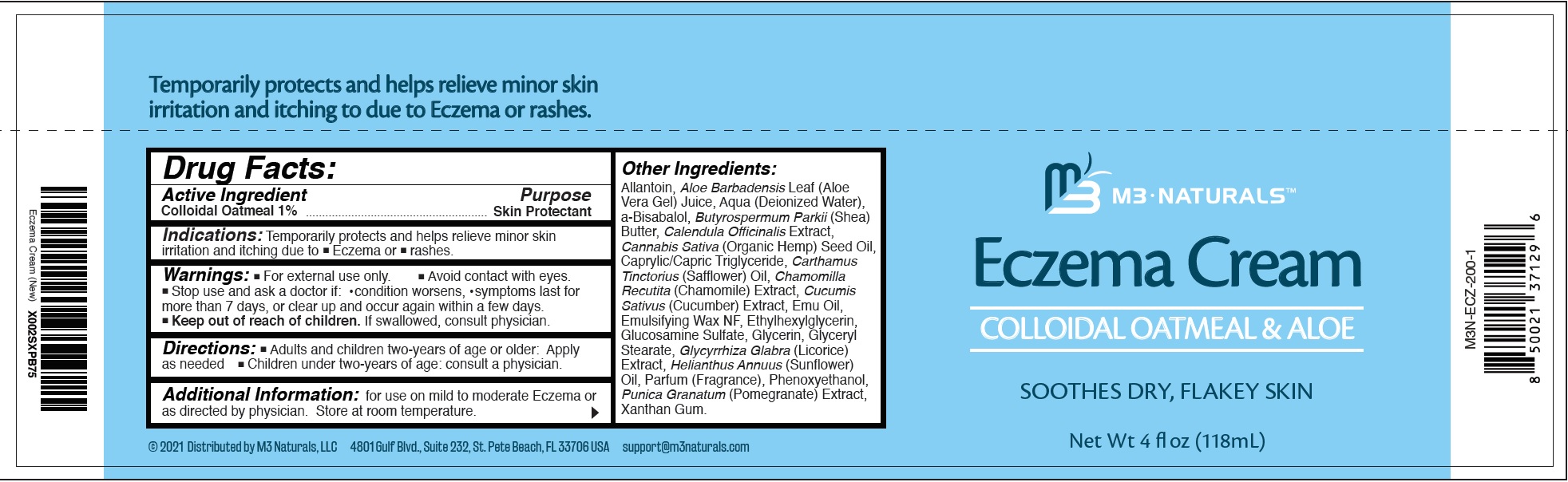
- Drug Facts:
- Active Ingredient
- Indications:
- Warnings:
- Directions:
- Additional Information:
-
Other Ingredients:
Allantoin, Aloe Barbadensis Leaf (Aloe Vera Gel) Juice, Aqua (Deionized Water), a-Bisabalol, Butyrospermum Parkii (Shea) Butter, Calendula Officinalis Extract, Cannabis Sativa (Organic Hemp) Seed Oil, Caprylic/Capric Triglyceride, Carthamus Tinctorius (Safflower) Oil, Chamomilla Recutita (Chamomile) Extract, Cucumis Sativus (Cucumber) Extract, Emu Oil, Emulsifying Wax NF, Ethylhexylglycerin, Glucosamine Sulfate, Glycerin, Glyceryl Stearate, Glycyrrhiza Glabra (Licorice) Extract, Helianthus Annuus (Sunflower) Oil, Parfum (Fragrance), Phenoxyethanol, Punica Granatum (Pomegranate) Extract, Xanthan Gum.
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
M3 NATURALS ECZEMA CREAM
oatmeal creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81985-186 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OATMEAL (UNII: 8PI54V663Y) (OATMEAL - UNII:8PI54V663Y) OATMEAL 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALLANTOIN (UNII: 344S277G0Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) SHEA BUTTER (UNII: K49155WL9Y) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) CANNABIS SATIVA SEED OIL (UNII: 69VJ1LPN1S) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CARTHAMUS TINCTORIUS FLOWER OIL (UNII: SDQ136WIM5) CHAMOMILE (UNII: FGL3685T2X) CUCUMBER (UNII: YY7C30VXJT) EMU OIL (UNII: 344821WD61) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLUCOSAMINE SULFATE (UNII: 1FW7WLR731) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) LICORICE (UNII: 61ZBX54883) HELIANTHUS ANNUUS FLOWERING TOP (UNII: BKJ0J3D1BP) PHENOXYETHANOL (UNII: HIE492ZZ3T) POMEGRANATE (UNII: 56687D1Z4D) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81985-186-01 118 mL in 1 JAR; Type 0: Not a Combination Product 04/19/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 04/19/2021 Labeler - M3 Naturals LLC (100802579)