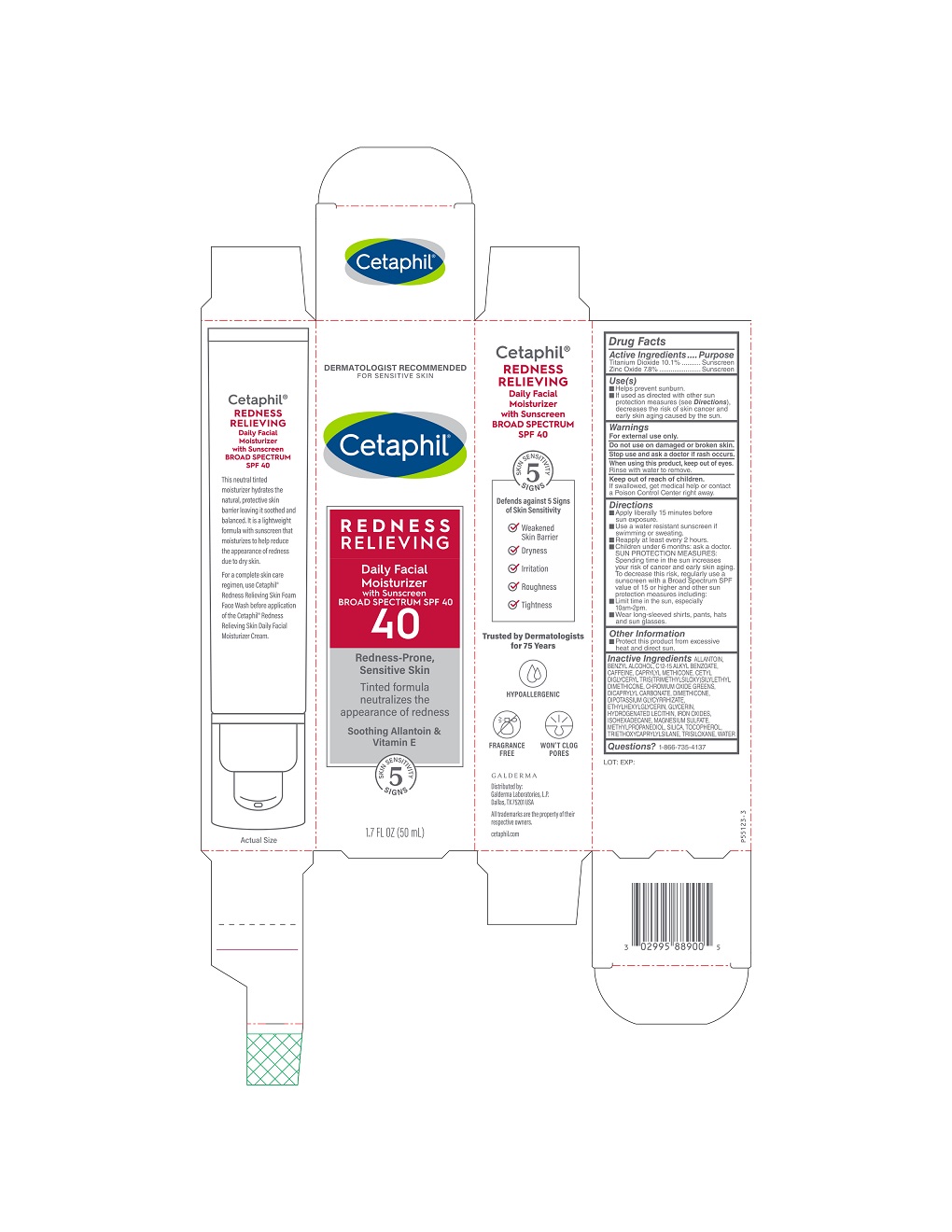
Label: CETAPHIL REDNESS RELIEVING DAILY FACIAL MOISTURIZER WITH SUNSCREEN SPF 40- titanium dioxide, zinc oxide cream
- NDC Code(s): 0299-4138-00, 0299-4138-05
- Packager: Galderma Laboratories, L.P.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Use(s)
- Warnings
- Keep out of reach of children.
-
Directions
• Apply liberally 15 minutes before sun exposure.
• Use a water resistant sunscreen if swimming or sweating
• Reapply at least every 2 hours.
• Children under 6 months: ask a doctor
SUN PROTECTION MEASURES: Spending time in the sun increases your risk of cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
• Limit time in the sun, especially 10am – 2 pm.
• Wear long-sleeved shirts, pants, hats, and sun glasses. - Other Information
-
Inactive Ingredients
ALLANTOIN, BENZYL ALCOHOL, C12-15 ALKYL BENZOATE, CAFFEINE, CAPRYLYL METHICONE, CETYL DIGLYCERYL TRIS(TRIMETHYLSILOXY) SILYLETHYL DIMETHICONE, CHROMIUM OXIDE GREENS, DICAPRYLYL CARBONATE, DIMETHICONE, DIPOTASSIUM GLYCYRRHIZATE, ETHYLHEXYLGLYCERIN, GLYCERIN, HYDROGENATED LECITHIN, IRON OXIDES, ISOHEXADECANE, MAGNESIUM SULFAGE, MEHYLPROPANEDIOL, SILICA, TOCOPHEROL, TRIETHOXYCAPRYLYLSILANE, TRISILOXANE, WATER
- Questions?
-
PRINCIPAL DISPLAY PANEL - 1.7 FL OZ (50 mL) carton
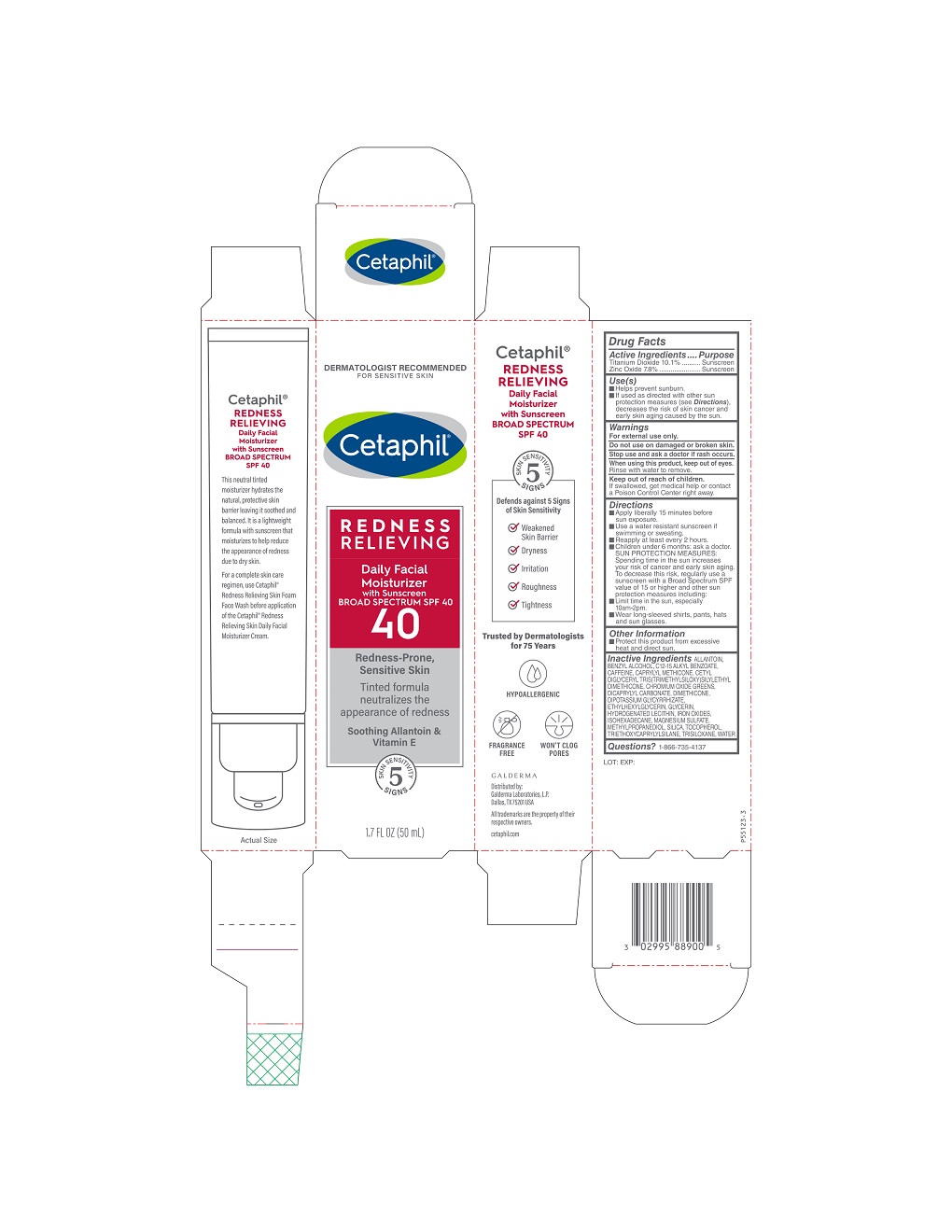
DERMATOLOGIST RECOMMENDED
FOR SENSITIVE SKIN
Cetaphil®
REDNESS
RELIEVING
Daily Facial
Moisturizer
with Sunscreen
BROAD SPECTRUM SPF 40
40
Redness-Prone,
Sensitive Skin
Tinted formula
neutralizes the
appearance of redness
Soothing Allantoin &
Vitamin E
5 Skin Sensitivity Signs
1.7 FL OZ (50 ML)Distributed by:
Galderma Laboratories, L.P.
Dallas, TX 75201 USA
All trademarks are the property of their
respective owners.
cetaphil.com
P55123-3
-
INGREDIENTS AND APPEARANCE
CETAPHIL REDNESS RELIEVING DAILY FACIAL MOISTURIZER WITH SUNSCREEN SPF 40
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0299-4138 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 101 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 78 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALLANTOIN (UNII: 344S277G0Z) BENZYL ALCOHOL (UNII: LKG8494WBH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CAFFEINE (UNII: 3G6A5W338E) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) CHROMIC OXIDE (UNII: X5Z09SU859) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERIN (UNII: PDC6A3C0OX) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) FERRIC OXIDE RED (UNII: 1K09F3G675) ISOHEXADECANE (UNII: 918X1OUF1E) Magnesium Sulfate, Unspecified Form (UNII: DE08037SAB) METHYLPROPANEDIOL (UNII: N8F53B3R4R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TOCOPHEROL (UNII: R0ZB2556P8) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) TRISILOXANE (UNII: 9G1ZW13R0G) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0299-4138-00 1 in 1 CARTON 07/20/2023 1 50 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:0299-4138-05 10 mL in 1 TUBE; Type 0: Not a Combination Product 07/20/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 07/20/2023 Labeler - Galderma Laboratories, L.P. (047350186) Establishment Name Address ID/FEI Business Operations Knowlton Development Corporation Inc. 204006464 manufacture(0299-4138)