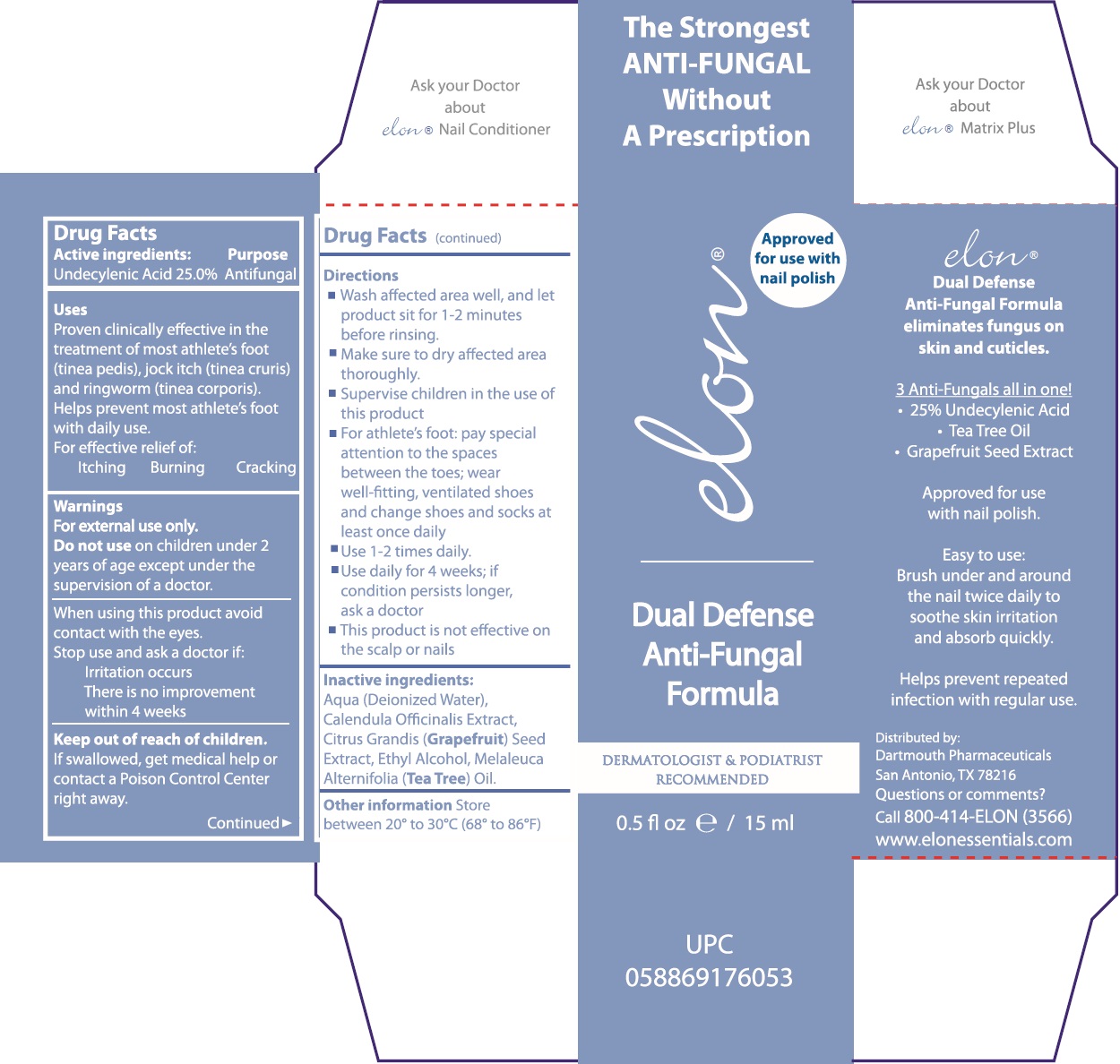
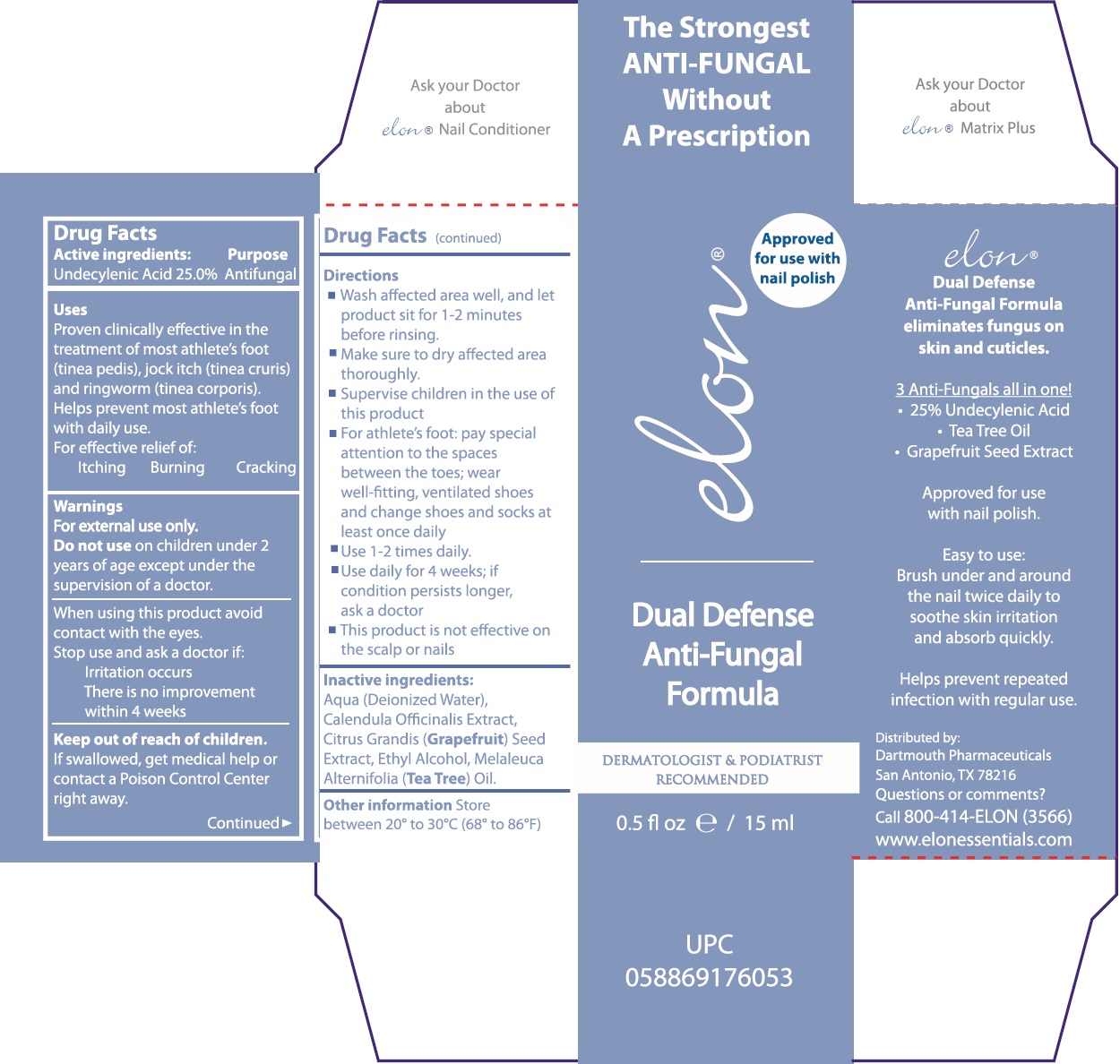
Label: ELON DUAL DEFENSE ANTI FUNGAL FORMULA- undecylenic acid liquid
- NDC Code(s): 71171-778-15
- Packager: Little Giant Holdings LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients:
- Uses
- Warnings
-
Directions
- Wash affected area well, and let product sit for 1-2 minutes before rinsing.
- Make sure to dry affected area thoroughly.
- Supervise children in the use of this product
- For athlete's foot: pay special attention to the spaces between the toes; wear well-fitting, ventilated shoes and change shoes and socks at leat once daily
- Use 1-2 times daily.
- Use daily for 4 weeks; if condition persists longer, ask a doctor
- This product is not effective on the scalp or nails
- Inactive ingredients:
- Other information
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
ELON DUAL DEFENSE ANTI FUNGAL FORMULA
undecylenic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71171-778 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength UNDECYLENIC ACID (UNII: K3D86KJ24N) (UNDECYLENIC ACID - UNII:K3D86KJ24N) UNDECYLENIC ACID 250 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) CITRUS MAXIMA SEED (UNII: 083X55C543) ALCOHOL (UNII: 3K9958V90M) TEA TREE OIL (UNII: VIF565UC2G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71171-778-15 1 in 1 BOX 01/02/2017 1 15 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 01/02/2017 Labeler - Little Giant Holdings LLC (080461006)