Label: BACITRACIN ZINC ointment
- NDC Code(s): 70677-1211-1
- Packager: STRATEGIC SOURCING SERVICES LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (each gram contains)
- Purpose
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
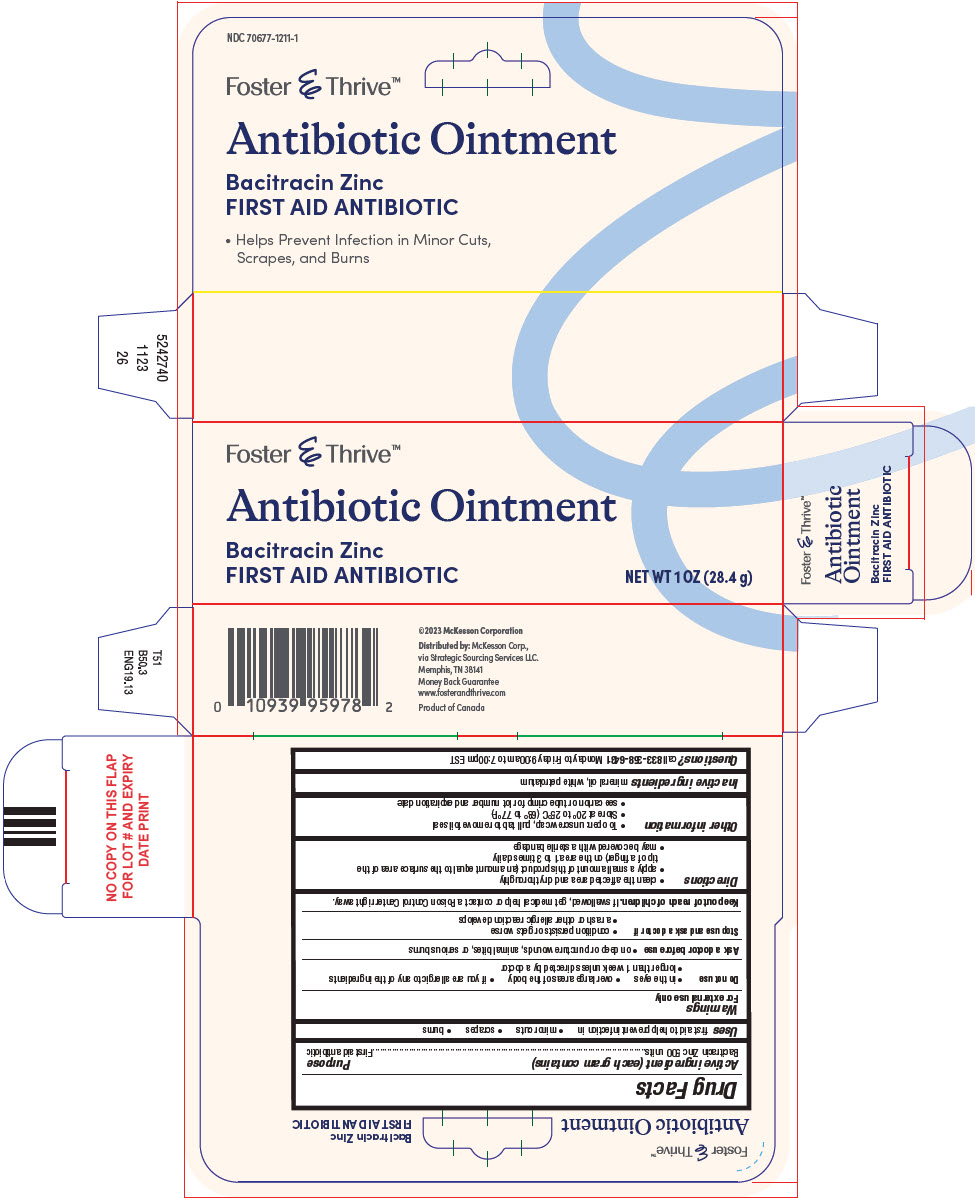
- PRINCIPAL DISPLAY PANEL - 28.4 g Tube Carton
-
INGREDIENTS AND APPEARANCE
BACITRACIN ZINC
bacitracin zinc ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70677-1211 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 500 [USP'U] in 1 g Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70677-1211-1 1 in 1 CARTON 12/15/2023 1 28.4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M004 12/15/2023 Labeler - STRATEGIC SOURCING SERVICES LLC (116956644) Establishment Name Address ID/FEI Business Operations Taro Pharmaceuticals Inc. 206263295 manufacture(70677-1211)