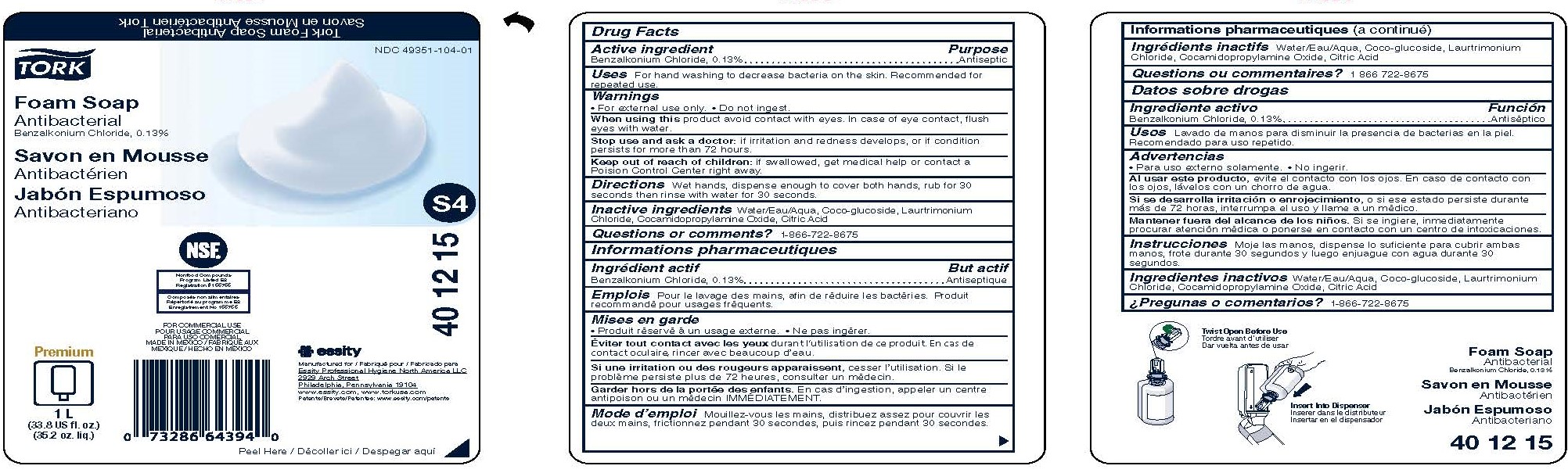
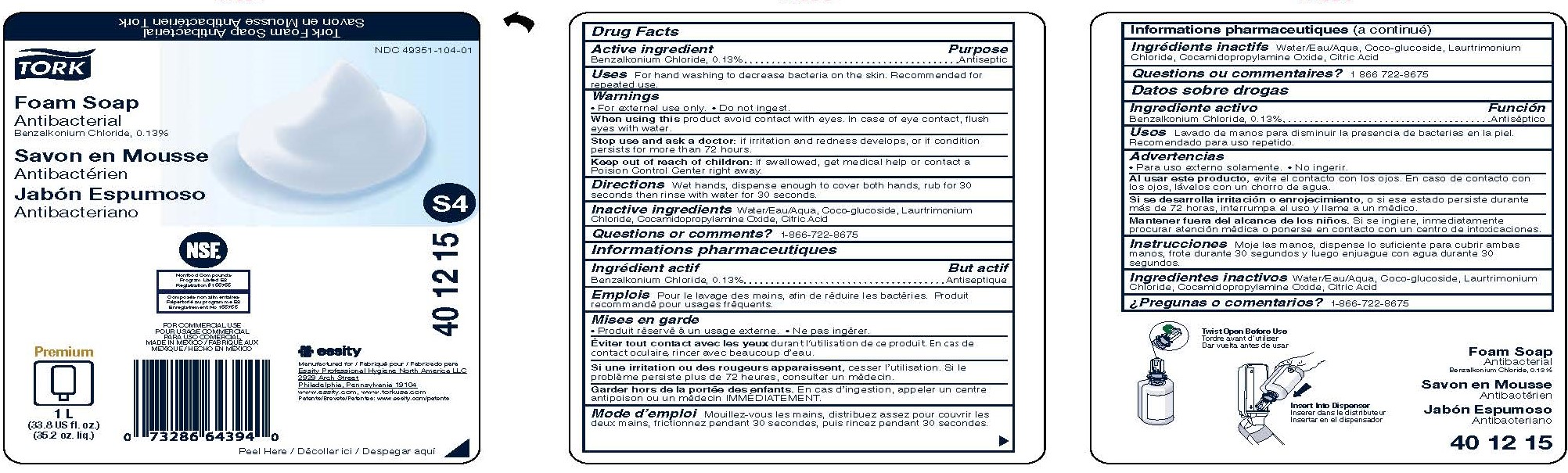
Label: TORK FOAM ANTIBACTERIAL- benzalkonium chloride soap
- NDC Code(s): 49351-104-01, 49351-104-02
- Packager: Essity Professional Hygiene North America LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated May 2, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Inactive ingredients
- Questions or Comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TORK FOAM ANTIBACTERIAL
benzalkonium chloride soapProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49351-104 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength COCO GLUCOSIDE (UNII: ICS790225B) LAURTRIMONIUM CHLORIDE (UNII: A81MSI0FIC) WATER (UNII: 059QF0KO0R) COCAMIDOPROPYLAMINE OXIDE (UNII: M4SL82J7HK) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49351-104-01 1000 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/15/2017 2 NDC:49351-104-02 24 in 1 BOX 03/15/2017 2 50 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 03/15/2017 Labeler - Essity Professional Hygiene North America LLC (005694349)