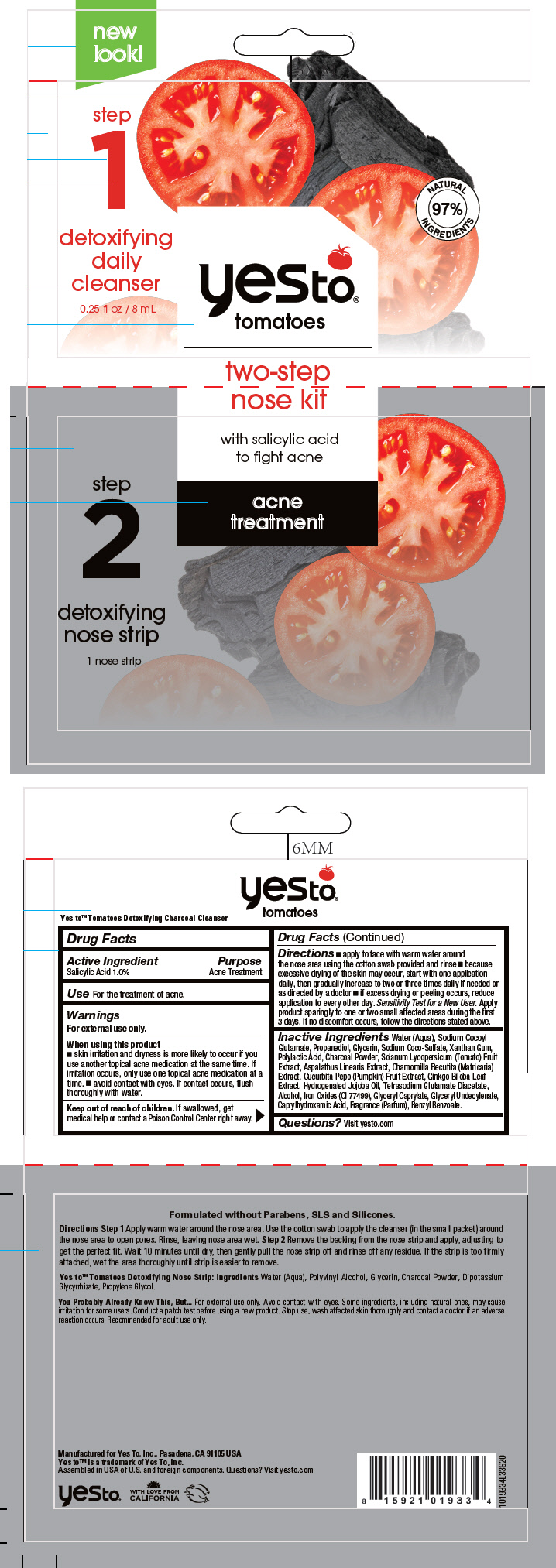
Label: YES TO TOMATOES TWO-STEP NOSE- salicylic acid kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 69840-025-16 - Packager: Yes To Incorporated
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 22, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Use
-
Warnings
For external use only.
-
Directions
- apply to face with warm water around the nose area using the cotton swab provided and rinse
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if excess drying or peeling occurs, reduce application to every other day.
-
Inactive Ingredients
Water (Aqua), Sodium Cocoyl Glutamate, Propanediol, Glycerin, Sodium Coco-Sulfate, Xanthan Gum, Polylactic Acid, Charcoal Powder, Solanum Lycopersicum (Tomato) Fruit Extract, Aspalathus Linearis Extract, Chamomilla Recutita (Matricaria) Extract, Cucurbita Pepo (Pumpkin) Fruit Extract, Ginkgo Biloba Leaf Extract, Hydrogenated Jojoba Oil, Tetrasodium Glutamate Diacetate, Alcohol, Iron Oxides (CI 77499), Glyceryl Caprylate, Glyceryl Undecylenate, Caprylhydroxamic Acid, Fragrance (Parfum), Benzyl Benzoate.
- Questions?
- PRINCIPAL DISPLAY PANEL - Kit Packet
-
INGREDIENTS AND APPEARANCE
YES TO TOMATOES TWO-STEP NOSE
salicylic acid kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69840-025 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69840-025-16 1 in 1 PACKET; Type 0: Not a Combination Product 01/15/2021 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 POUCH 8 mL Part 2 1 POUCH 1 Part 1 of 2 DETOXIFYING DAILY CLEANSER
salicylic acid liquidProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Salicylic Acid (UNII: O414PZ4LPZ) (Salicylic Acid - UNII:O414PZ4LPZ) Salicylic Acid 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength XANTHAN GUM (UNII: TTV12P4NEE) GLYCERIN (UNII: PDC6A3C0OX) SODIUM COCOYL GLUTAMATE (UNII: BMT4RCZ3HG) SODIUM COCO-SULFATE (UNII: 3599J29ANH) GLYCERYL MONOCAPRYLATE (UNII: TM2TZD4G4A) GLYCERYL 1-UNDECYLENATE (UNII: B68LJT9544) PROPANEDIOL (UNII: 5965N8W85T) GINKGO (UNII: 19FUJ2C58T) CHAMOMILE (UNII: FGL3685T2X) ASPALATHUS LINEARIS WHOLE (UNII: O17JQA1A9Z) CUCURBITA PEPO WHOLE (UNII: 48V44WX64I) SOLANUM LYCOPERSICUM FRUITING TOP (UNII: X636CG4BH0) ALCOHOL (UNII: 3K9958V90M) ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) HYDROGENATED JOJOBA OIL (UNII: 7F674YQ5SO) POLYLACTIDE (UNII: 459TN2L5F5) FERRIC OXIDE RED (UNII: 1K09F3G675) WATER (UNII: 059QF0KO0R) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) Product Characteristics Color BLACK Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 8 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part333D 01/15/2021 Part 2 of 2 DETOXIFYING NOSE STRIP
other skin care preparations patchProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR Water (UNII: 059QF0KO0R) INGR POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) INGR GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) INGR PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Product Characteristics color BLACK C48323 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date COSMETIC 01/15/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 01/15/2021 Labeler - Yes To Incorporated (788689680) Establishment Name Address ID/FEI Business Operations Bentley Laboratories LLC 068351753 MANUFACTURE(69840-025)