Label: THE INDIGO CREAM- colloidal oatmeal 2% cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 69417-120-03, 69417-120-17 - Packager: TATCHA, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 31, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Inactive ingredients
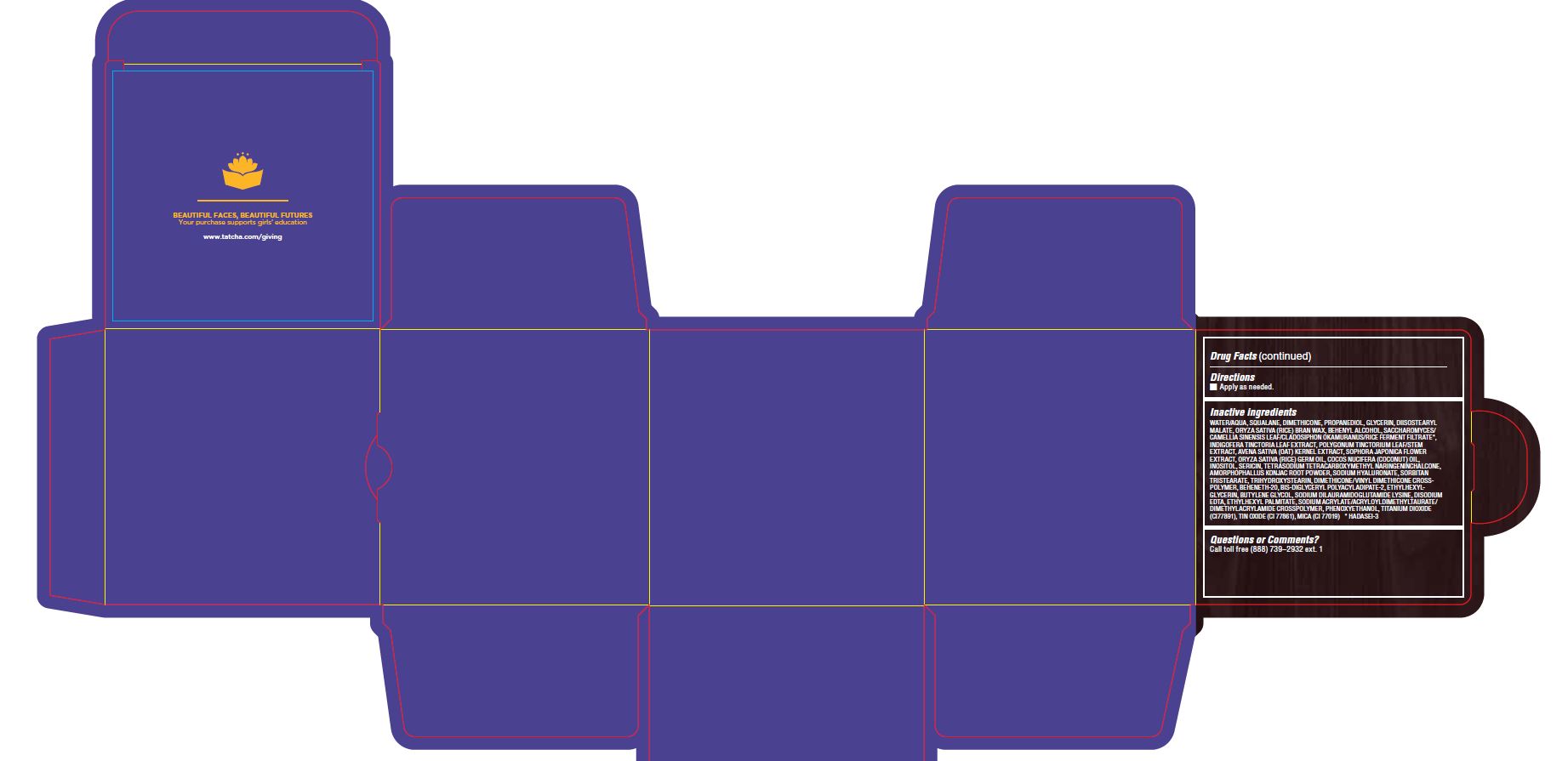
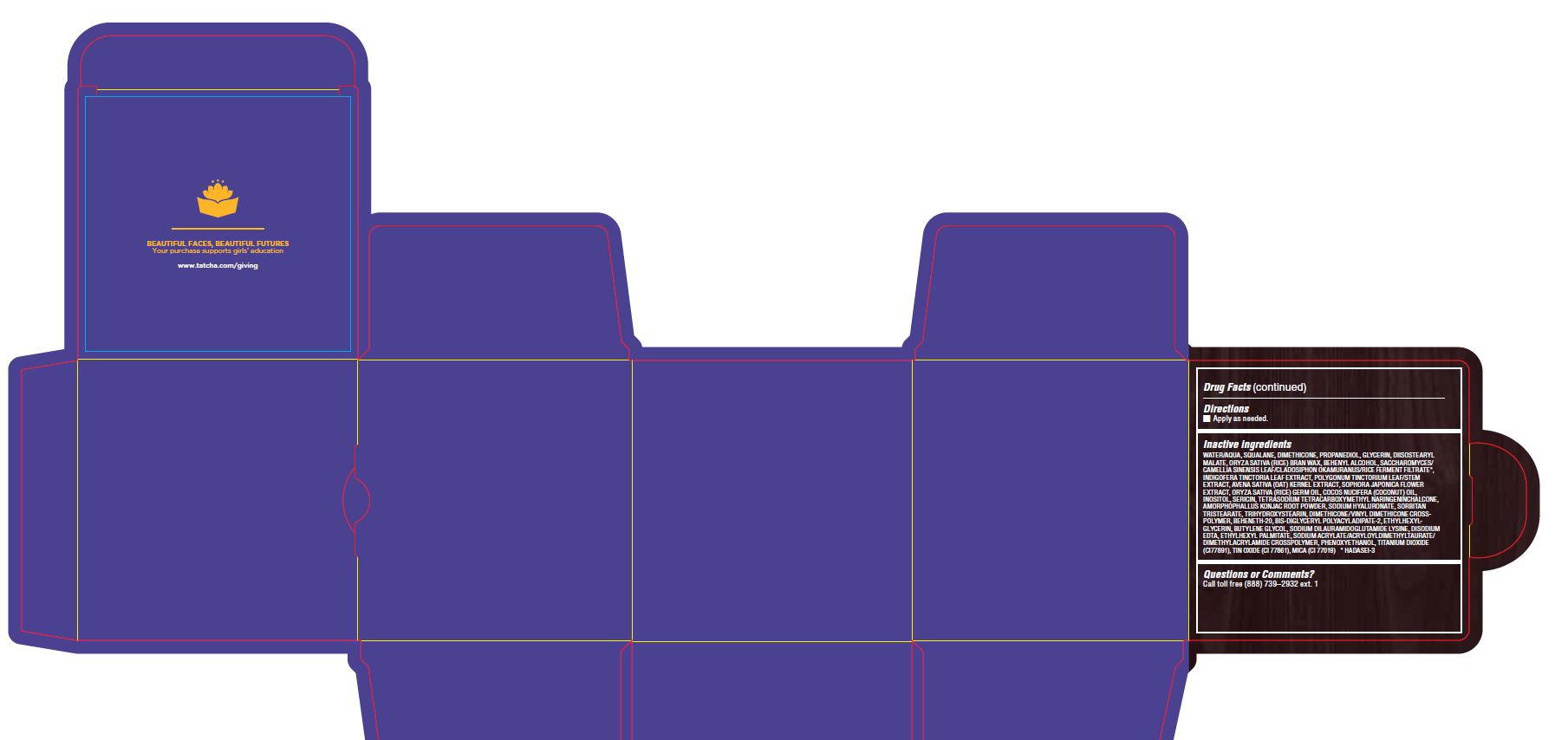
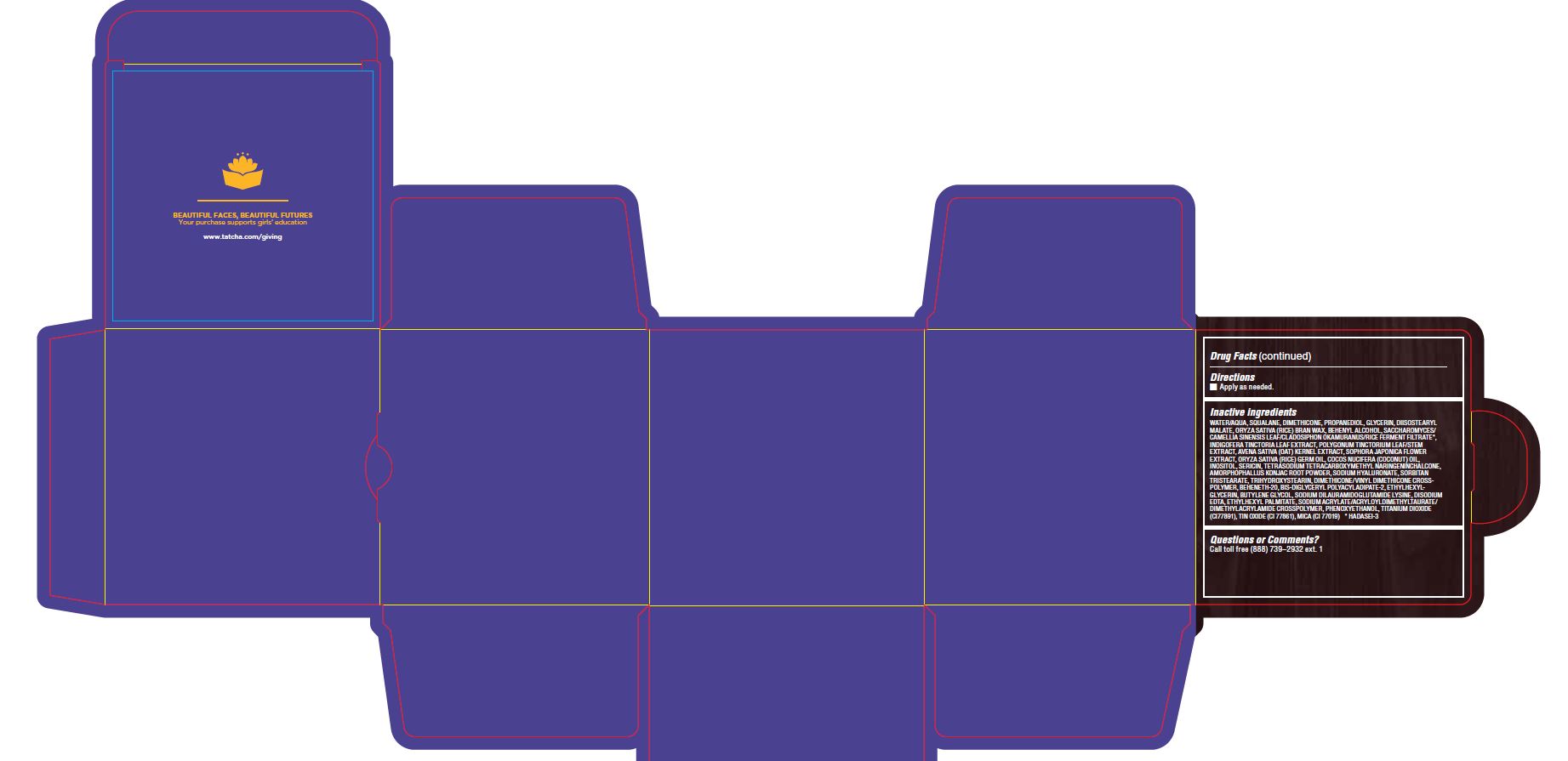
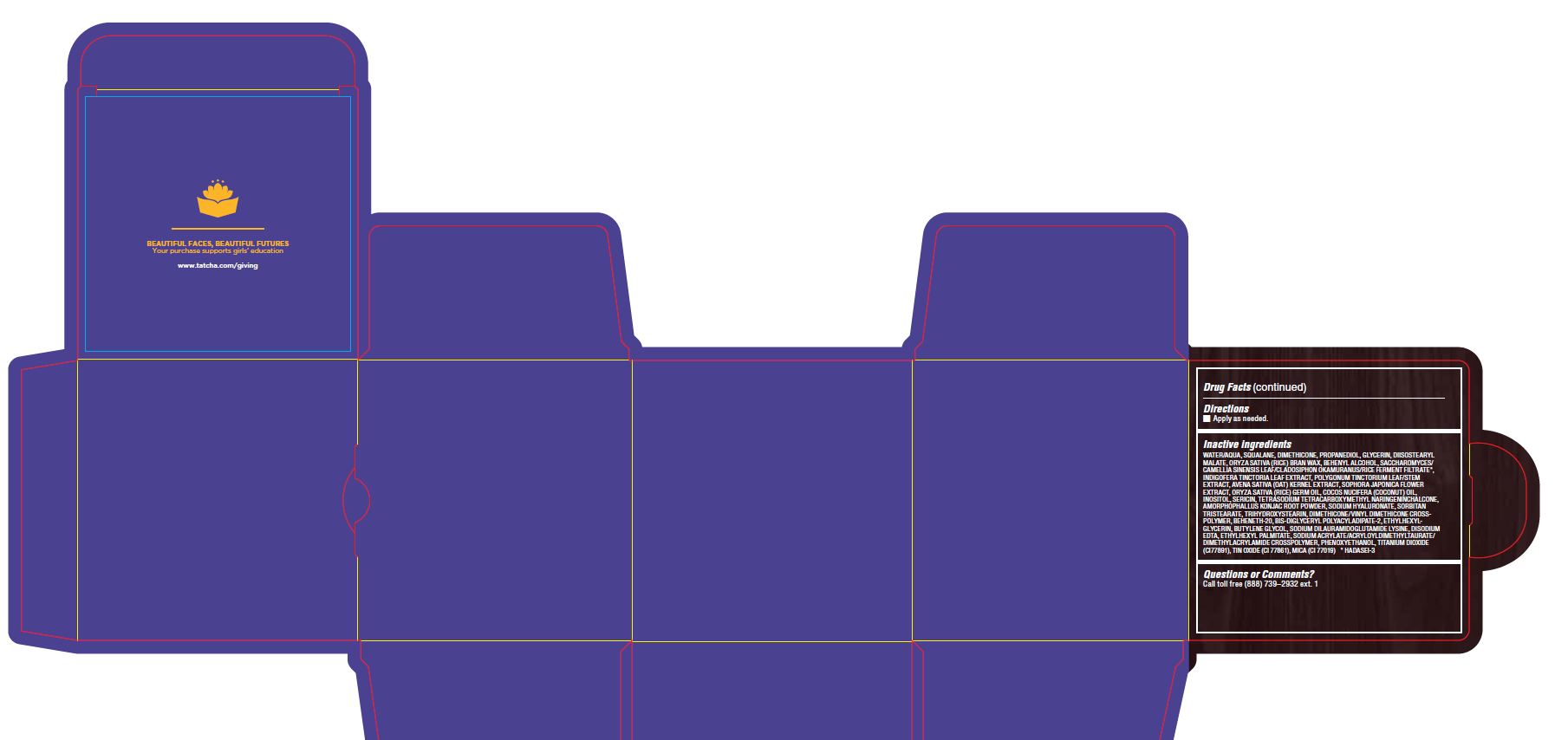
WATER/AQUA, SQUALANE, DIMETHICONE, PROPANEDIOL, GLYCERIN, DIISOSTEARYL MALATE, ORYZA SATIVA (RICE) BRAN WAX, BEHENYL ALCOHOL, SACCHAROMYCES/CAMELLIA SINENSIS LEAF/CLADOSIPHON OKAMURANUS/RICE FERMENT FILTRATE*, INDIGOFERA TINCTORIA LEAF EXTRACT, POLYGONUM TINCTORIUM LEAF/STEM EXTRACT, AVENA SATIVA (OAT) KERNEL EXTRACT, SOPHORA JAPONICA FLOWER EXTRACT, ORYZA SATIVA (RICE) GERM OIL, COCOS NUCIFERA (COCONUT) OIL, INOSITOL, SERICIN, TETRASODIUM TETRACARBOXYMETHYL NARINGENINCHALCONE, AMORPHOPHALLUS KONJAC ROOT POWDER, SODIUM HYALURONATE, SORBITAN TRISTEARATE, TRIHYDROXYSTEARIN, DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER, BEHENETH-20, BIS-DIGLYCERYL POLYACYLADIPATE-2, ETHYLHEXYLGLYCERIN, BUTYLENE GLYCOL, SODIUM DILAURAMIDOGLUTAMIDE LYSINE, DISODIUM EDTA, ETHYLHEXYL PALMITATE, SODIUM ACRYLATE/ACRYLOYLDIMETHYLTAURATE/ DIMETHYLACRYLAMIDE CROSSPOLYMER, PHENOXYETHANOL, TITANIUM DIOXIDE (CI77891), TIN OXIDE (CI 77861), MICA (CI 77019) * HADASEI-3
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
THE INDIGO CREAM
colloidal oatmeal 2% creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69417-120 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OATMEAL (UNII: 8PI54V663Y) (OATMEAL - UNII:8PI54V663Y) OATMEAL 2 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SQUALANE (UNII: GW89575KF9) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PROPANEDIOL (UNII: 5965N8W85T) GLYCERIN (UNII: PDC6A3C0OX) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) RICE BRAN (UNII: R60QEP13IC) DOCOSANOL (UNII: 9G1OE216XY) CAMELLIA SINENSIS WHOLE (UNII: C5M4585ZBZ) INDIGOFERA TINCTORIA LEAF (UNII: 2K7DF8HZDM) PERSICARIA TINCTORIA LEAF (UNII: FU6582QMPV) OAT KERNEL OIL (UNII: 3UVP41R77R) STYPHNOLOBIUM JAPONICUM FLOWER (UNII: 644C3CSB6E) RICE GERM OIL (UNII: D4H5GGS5JF) COCONUT OIL (UNII: Q9L0O73W7L) INOSITOL (UNII: 4L6452S749) SERICIN 1 (UNII: N6466G03F7) AMORPHOPHALLUS KONJAC ROOT (UNII: F7KU2UY3HE) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SORBITAN TRISTEARATE (UNII: 6LUM696811) TRIHYDROXYSTEARIN (UNII: 06YD7896S3) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) BEHENETH-20 (UNII: BJ4GP2IFLN) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DISODIUM EDTA-COPPER (UNII: 6V475AX06U) ETHYLHEXYL PALMITATE (UNII: 2865993309) SODIUM ACRYLATE (UNII: 7C98FKB43H) PHENOXYETHANOL (UNII: HIE492ZZ3T) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) STANNIC OXIDE (UNII: KM7N50LOS6) MICA (UNII: V8A1AW0880) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69417-120-17 1 in 1 BOX 08/02/2018 1 50 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:69417-120-03 1 in 1 BOX 08/02/2018 2 10 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 08/02/2018 Labeler - TATCHA, Inc. (006811461) Establishment Name Address ID/FEI Business Operations Thibiant International, Inc. 083913913 manufacture(69417-120)