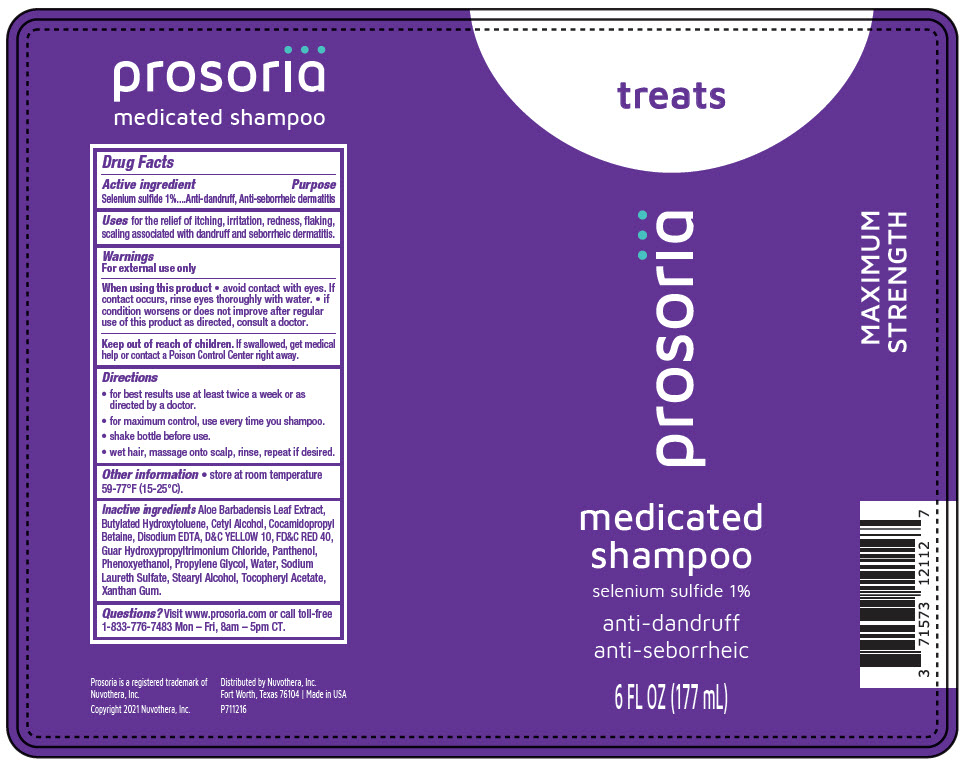
Label: PROSORIA MEDICATED- selenium sulfide lotion/shampoo
- NDC Code(s): 71573-121-12
- Packager: Nuvothera, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
Aloe Barbadensis Leaf Extract, Butylated Hydroxytoluene, Cetyl Alcohol, Cocamidopropyl Betaine, Disodium EDTA, D&C YELLOW 10, FD&C RED 40, Guar Hydroxypropyltrimonium Chloride, Panthenol, Phenoxyethanol, Propylene Glycol, Water, Sodium Laureth Sulfate, Stearyl Alcohol, Tocopheryl Acetate, Xanthan Gum.
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 177 mL Bottle Label
-
INGREDIENTS AND APPEARANCE
PROSORIA MEDICATED
selenium sulfide lotion/shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71573-121 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Selenium Sulfide (UNII: Z69D9E381Q) (Selenium Sulfide - UNII:Z69D9E381Q) Selenium Sulfide 1.77 g in 177 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Cetyl alcohol (UNII: 936JST6JCN) GUAR HYDROXYPROPYLTRIMONIUM CHLORIDE (1.7 SUBSTITUENTS PER SACCHARIDE) (UNII: B16G315W7A) Aloe Vera Leaf (UNII: ZY81Z83H0X) Butylated Hydroxytoluene (UNII: 1P9D0Z171K) Cocamidopropyl Betaine (UNII: 5OCF3O11KX) D&C Yellow No. 10 (UNII: 35SW5USQ3G) Edetate Disodium Anhydrous (UNII: 8NLQ36F6MM) FD&C Red No. 40 (UNII: WZB9127XOA) Panthenol (UNII: WV9CM0O67Z) Propylene Glycol (UNII: 6DC9Q167V3) Stearyl Alcohol (UNII: 2KR89I4H1Y) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Xanthan Gum (UNII: TTV12P4NEE) Sodium Laureth-3 Sulfate (UNII: BPV390UAP0) Phenoxyethanol (UNII: HIE492ZZ3T) Product Characteristics Color ORANGE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71573-121-12 177 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M032 04/01/2022 Labeler - Nuvothera, Inc. (080499864) Establishment Name Address ID/FEI Business Operations Quality CDMO 117658386 MANUFACTURE(71573-121)