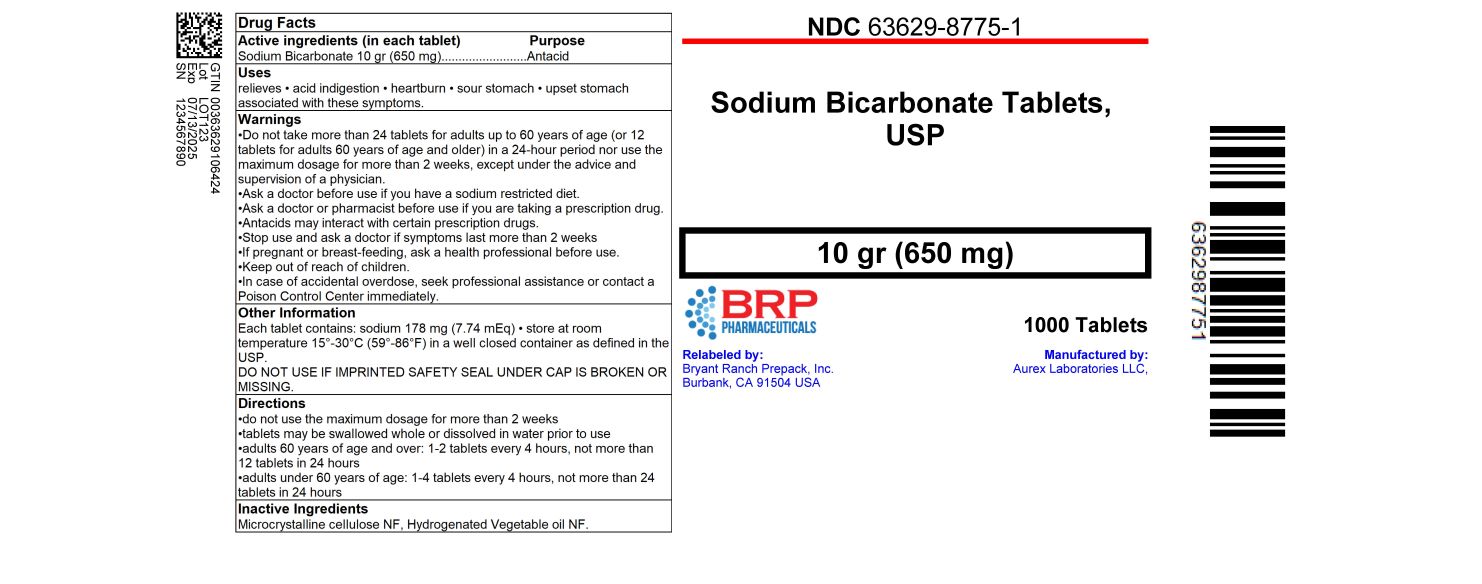
Label: SODIUM BICARBONATE tablet
- NDC Code(s): 63629-8775-1
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 64980-528
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- BOXED WARNING (What is this?)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Do not take more than 24 tablets for adults up to 60 years of age (or 12 tablets for adults 60 years of age and older) in a 24-hour period nor use the maximum dosage for more than 2 weeks, except under the advice and supervision of a physician.
Ask a doctor before use if you have a sodium restricted diet.
Ask a doctor or pharmacist before use if you are taking a prescription drug.
Antacids may interact with certain prescription drugs.
Stop use and ask a doctor if symptoms last more than 2 weeks
If pregnant or breast-feeding, ask a health professional before use. - KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
- do not use the maximum dosage for more than 2 weeks
- tablets may be swallowed whole or dissolved in water prior to use
- adults 60 years of age and over: 1-2 tablets every 4 hours, not more than 12 tablets in 24 hours
- adults under 60 years of age: 1-4 tablets every 4 hours, not more than 24 tablets in 24 hours
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
- SPL UNCLASSIFIED SECTION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SODIUM BICARBONATE
sodium bicarbonate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63629-8775(NDC:64980-528) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM BICARBONATE (UNII: 8MDF5V39QO) (BICARBONATE ION - UNII:HN1ZRA3Q20) SODIUM BICARBONATE 650 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CORN OIL (UNII: 8470G57WFM) Product Characteristics Color WHITE (White to off white) Score no score Shape ROUND Size 11mm Flavor Imprint Code AS3 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63629-8775-1 1000 in 1 BOTTLE; Type 0: Not a Combination Product 08/17/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M001 07/20/2020 Labeler - Bryant Ranch Prepack (171714327) Registrant - Bryant Ranch Prepack (171714327) Establishment Name Address ID/FEI Business Operations Bryant Ranch Prepack 171714327 REPACK(63629-8775) , RELABEL(63629-8775)