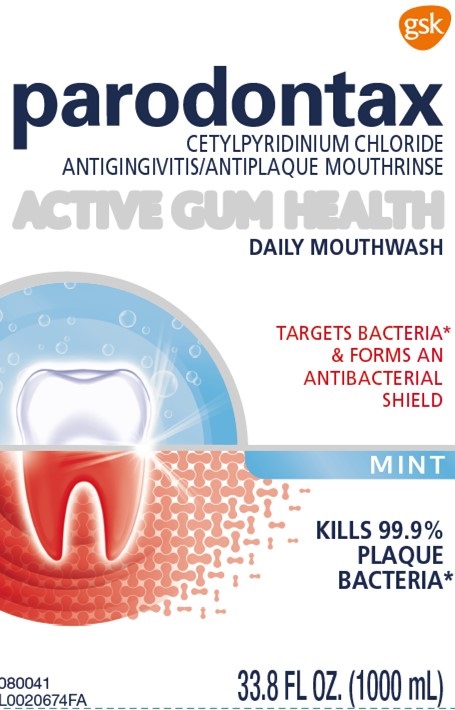
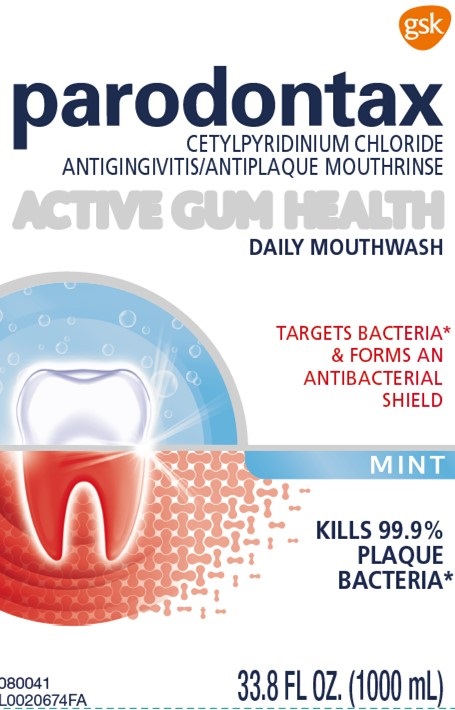
Label: PARODONTAX ACTIVE GUM HEALTH MINT- cetylpyridinium chloride mouthwash
PARODONTAX ACTIVE GUM HEALTH CLEAR MINT- cetylpyridinium chloride mouthwash
- NDC Code(s): 0135-0650-01, 0135-0651-02, 0135-0651-03
- Packager: Haleon US Holdings LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
Stop use and ask a dentist if
- irritation occurs
- gingivitis, bleeding, or redness persists for more than 2 weeks
- you have painful or swollen gums, pus from the gum line, loose teeth, or increasing spacing between the teeth. These may be signs or symptoms of periodontitis, a serious form of gum disease.
-
Directions
- adults and children 6 years of age and older:vigorously swish 20 milliliters (4 teaspoonfuls) of the rinse between your teeth twice a day for 30 seconds and then spit out. Do not swallow the rinse. Do not rinse with water. Do not drink from the bottle.
- children 6 years to under 12 years of age:supervisor use
- children under 6 years of age:do not use
- Other information
- Inactive ingredients (Mint)
- Inactive Ingredients (Clear Mint)
- Questions or comments?
-
Additional Information
*Kills plaque bacteria associated with gingivitis and odor-causing bacteria in a laboratory test
Do not use if the printed seal on the cap is broken or missing.
ALWAYS FOLLOW THE LABEL
Distributed by: GSK Consumer Healthcare,Warren, NJ 07059
Trademarks are owned by or licensed to the GSK group of companies.
©2022 GSK group of companies or its licensor.
1-855-328-5202
- Principal Display Panel
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
PARODONTAX ACTIVE GUM HEALTH MINT
cetylpyridinium chloride mouthwashProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0135-0651 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) (CETYLPYRIDINIUM - UNII:CUB7JI0JV3) CETYLPYRIDINIUM CHLORIDE 70 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) POLOXAMER 188 (UNII: LQA7B6G8JG) SACCHARIN SODIUM (UNII: SB8ZUX40TY) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM BENZOATE (UNII: OJ245FE5EU) SUCRALOSE (UNII: 96K6UQ3ZD4) 2,3'-IMINODI-BENZOIC ACID (UNII: H1C37ZB0P3) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0135-0651-02 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2022 2 NDC:0135-0651-03 1000 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/03/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M022 01/03/2022 PARODONTAX ACTIVE GUM HEALTH CLEAR MINT
cetylpyridinium chloride mouthwashProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0135-0650 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) (CETYLPYRIDINIUM - UNII:CUB7JI0JV3) CETYLPYRIDINIUM CHLORIDE 0.7 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) POLOXAMER 188 (UNII: LQA7B6G8JG) SACCHARIN SODIUM (UNII: SB8ZUX40TY) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM BENZOATE (UNII: OJ245FE5EU) SUCRALOSE (UNII: 96K6UQ3ZD4) BENZOIC ACID (UNII: 8SKN0B0MIM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0135-0650-01 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M022 01/03/2022 Labeler - Haleon US Holdings LLC (079944263)