Label: YOUNIQUE ROYALTY HYDRATING DAY CREAM SPF 20- avobenzone, homosalate, octisalate, octocrylene lotion
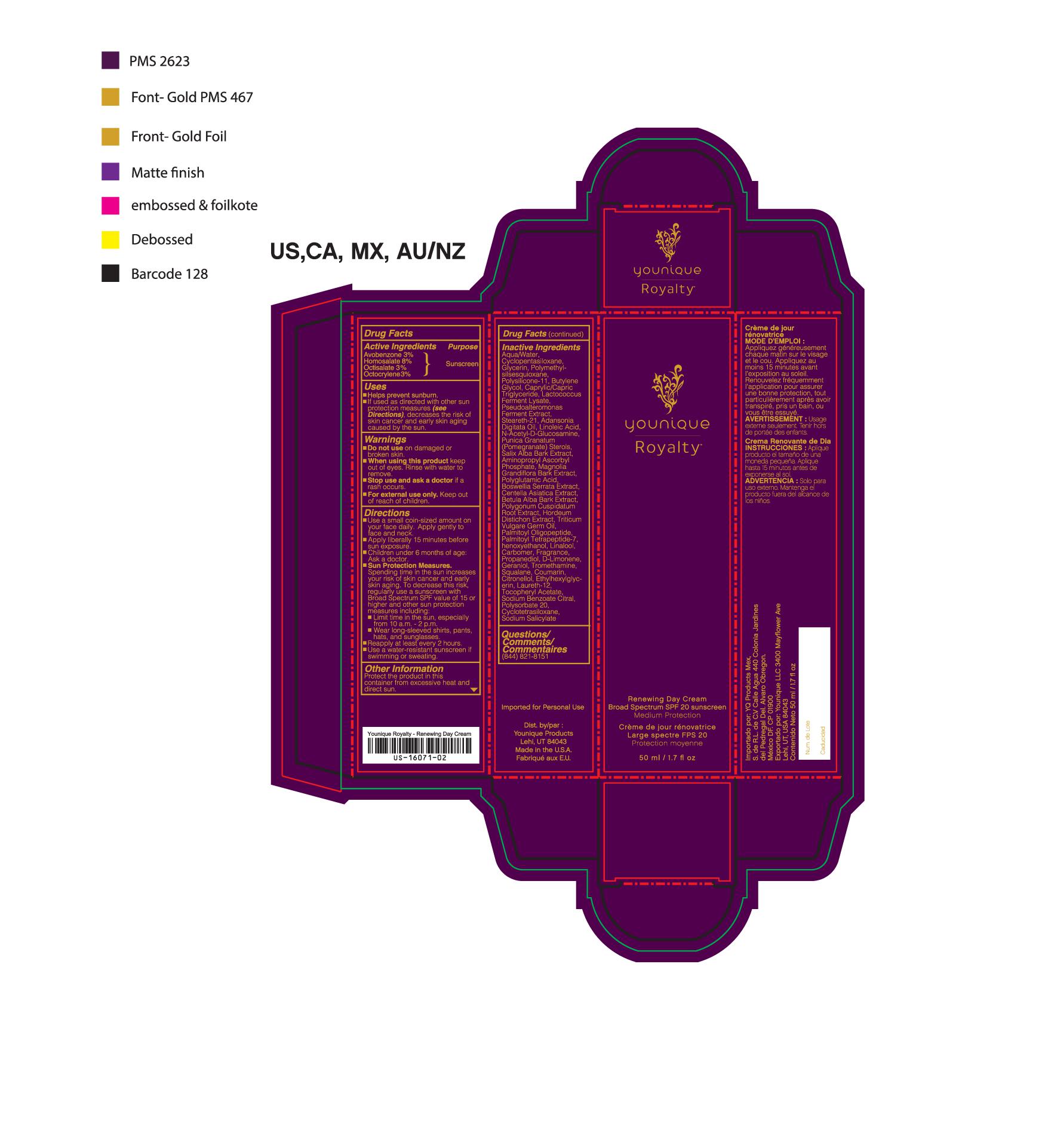
YOUNIQUE ROYALTY RENEWING DAY CREAM SPF 20- avobenzone, homosalate, octisalate, octocrylene lotion
- NHRIC Code(s): 71182-3550-1, 71182-3440-1
- Packager: Younique LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Cosmetic
Drug Label Information
Updated November 13, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
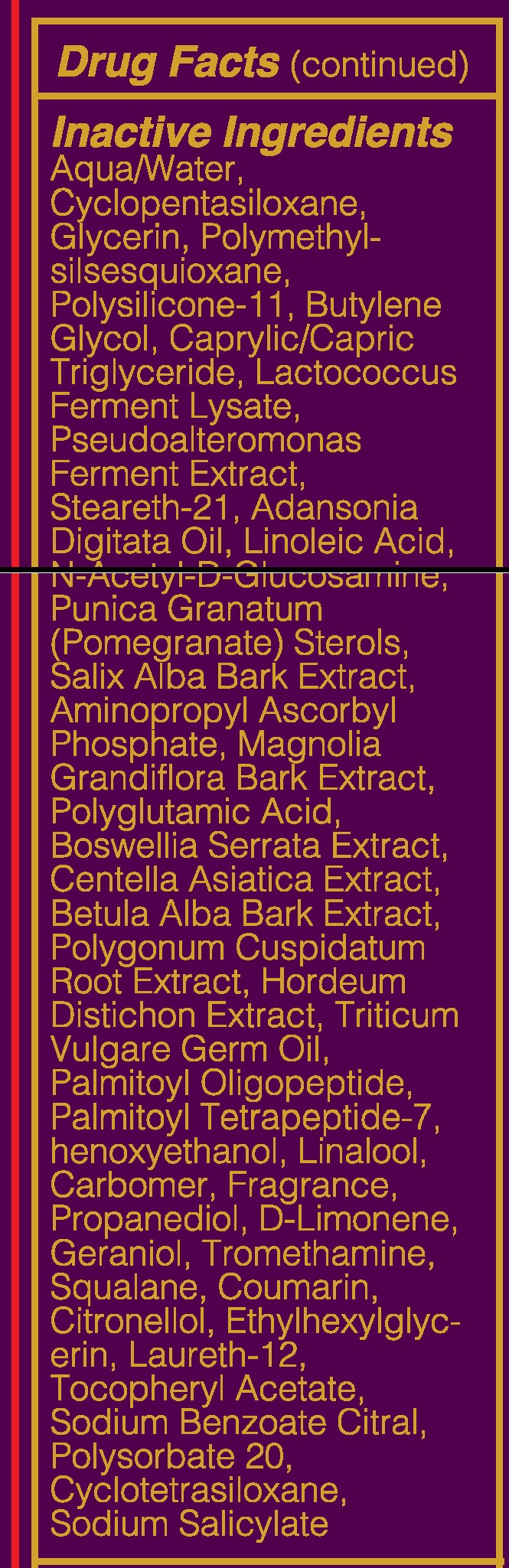
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
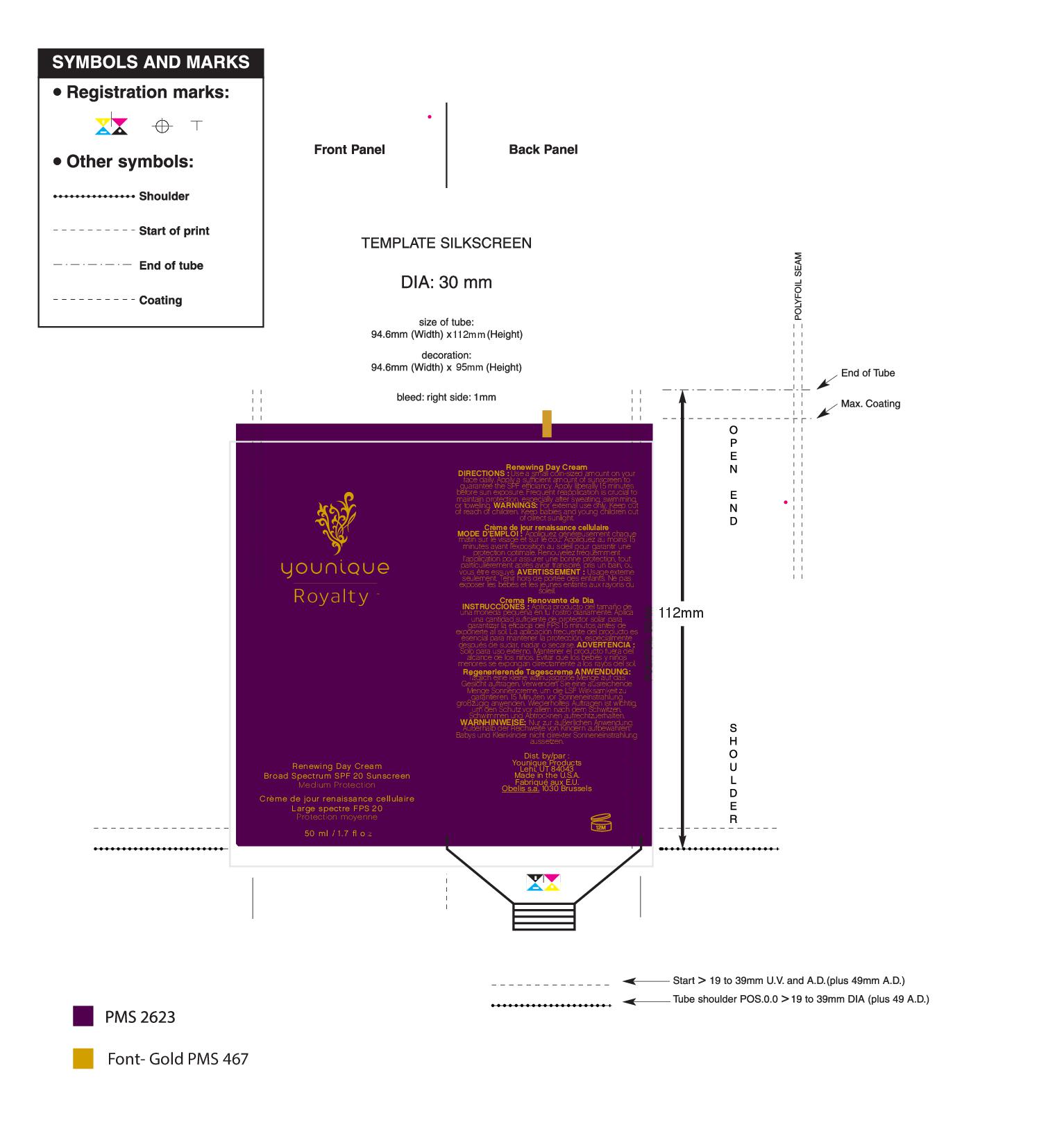
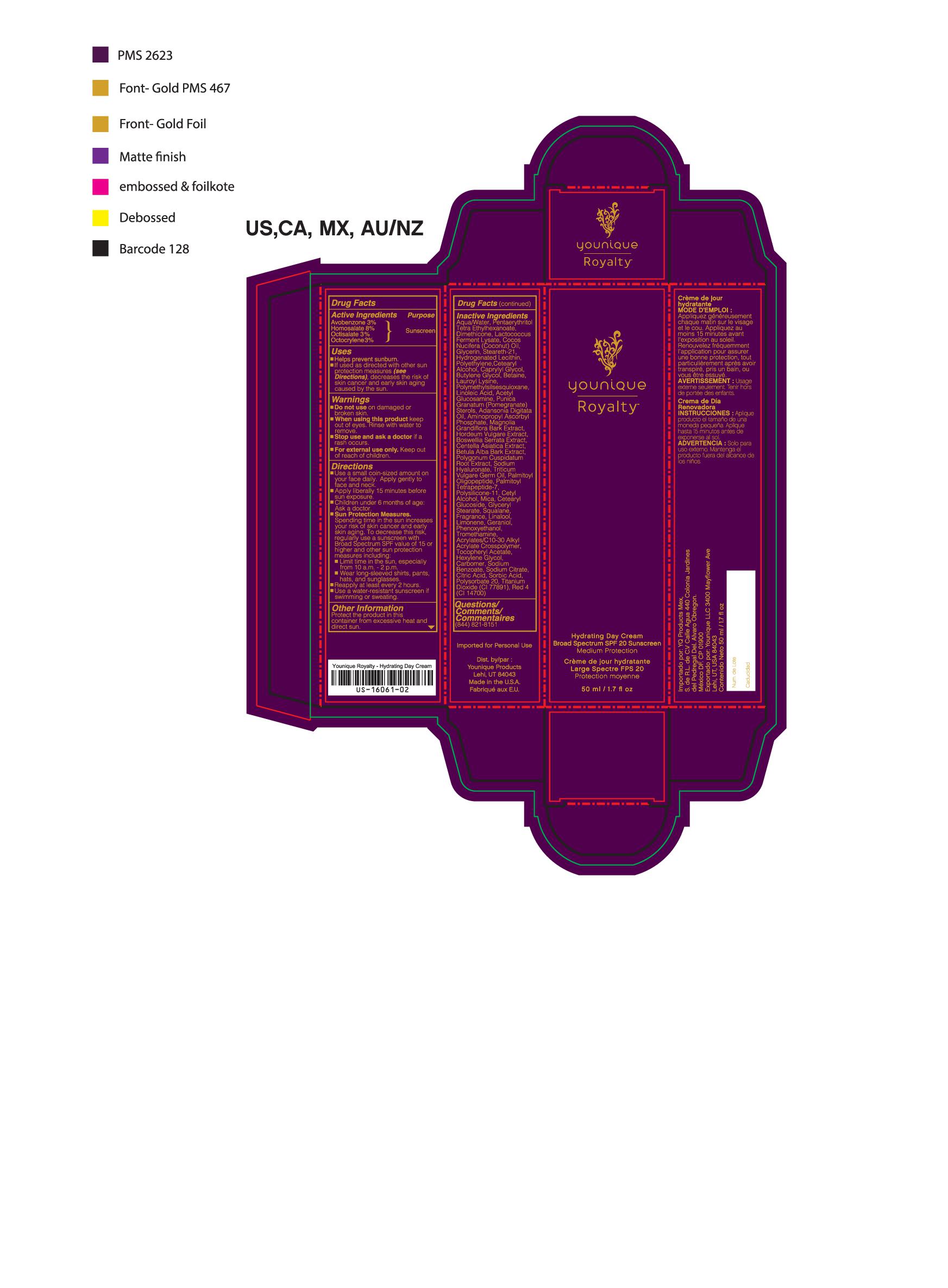
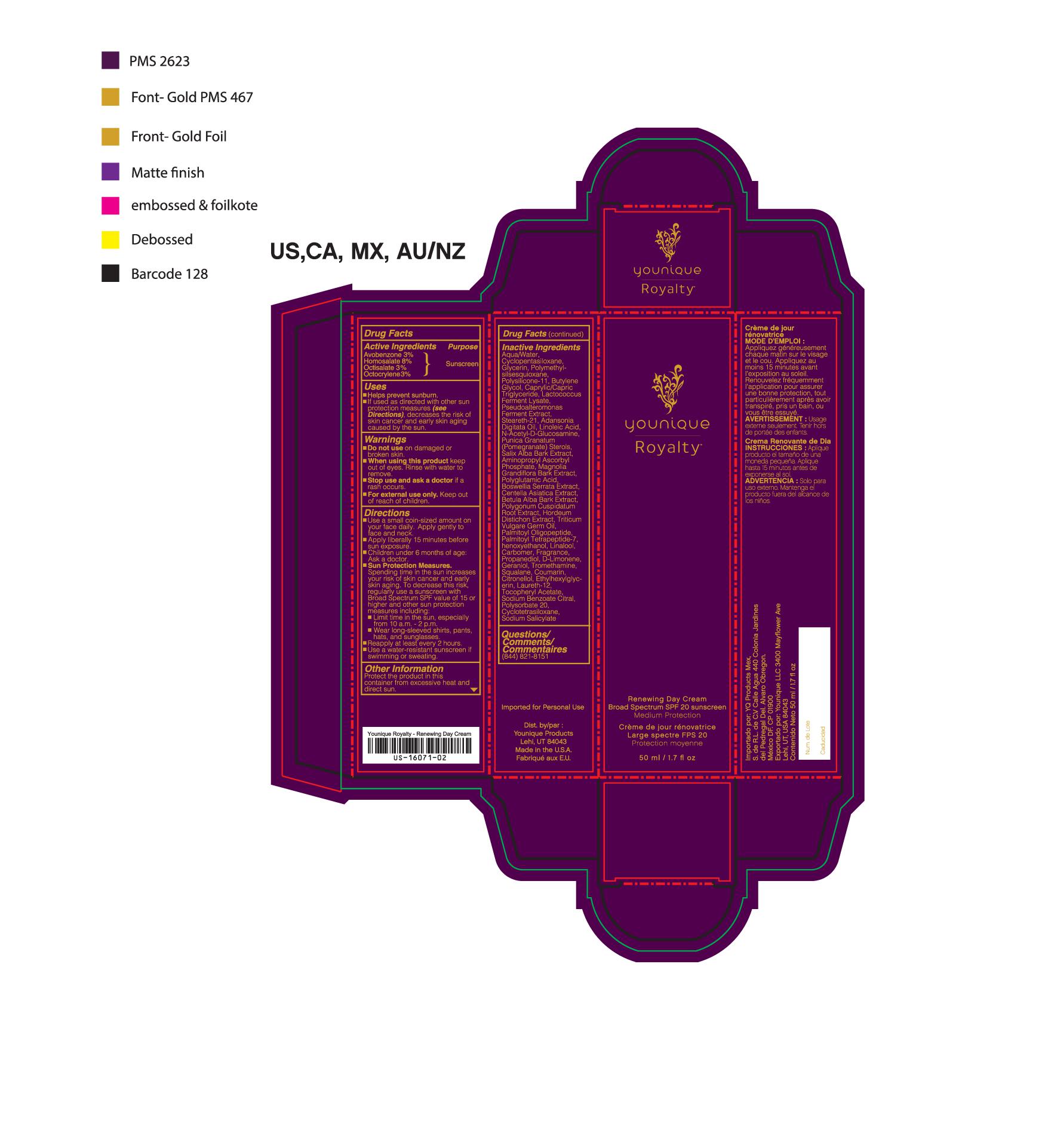
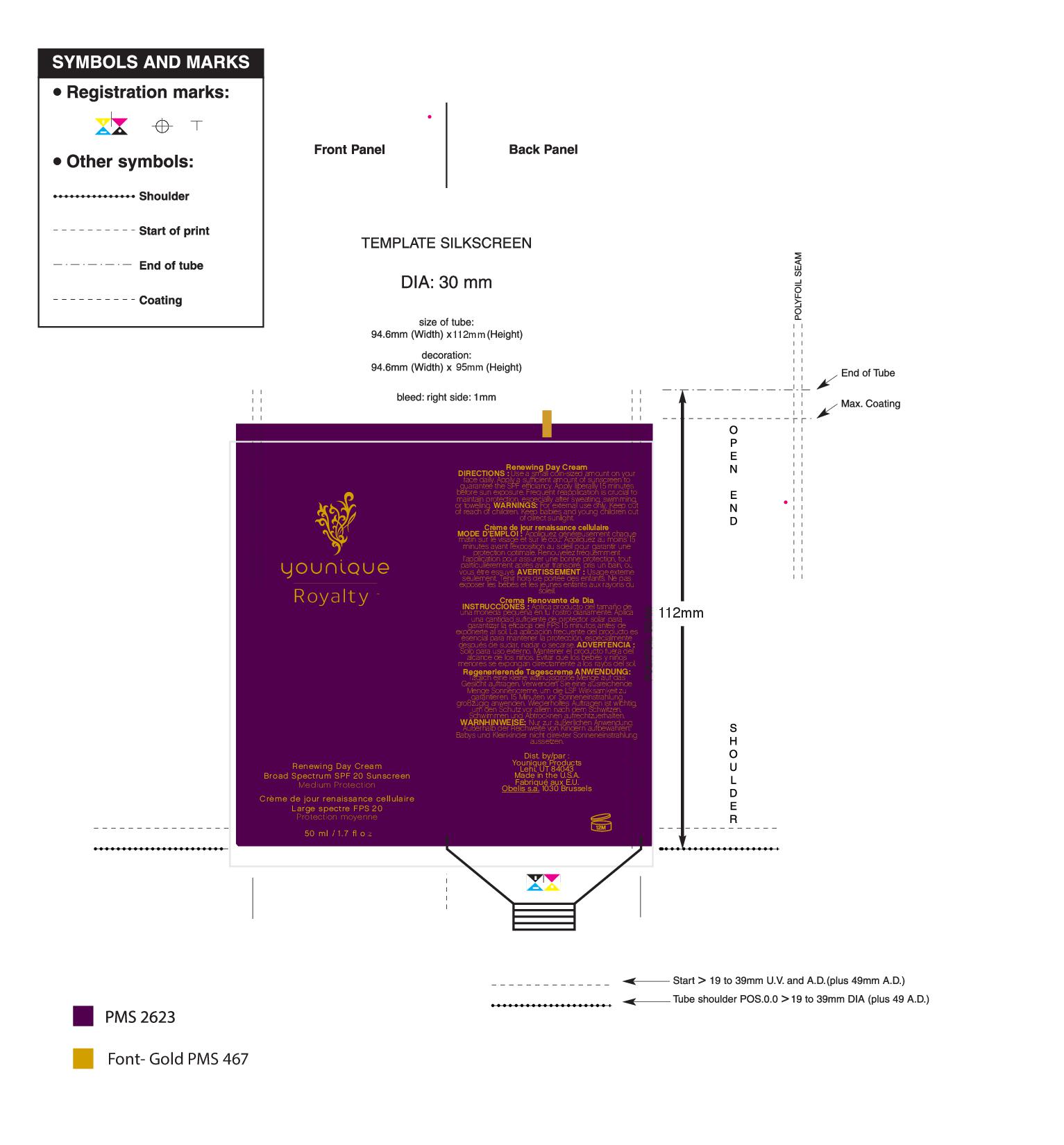
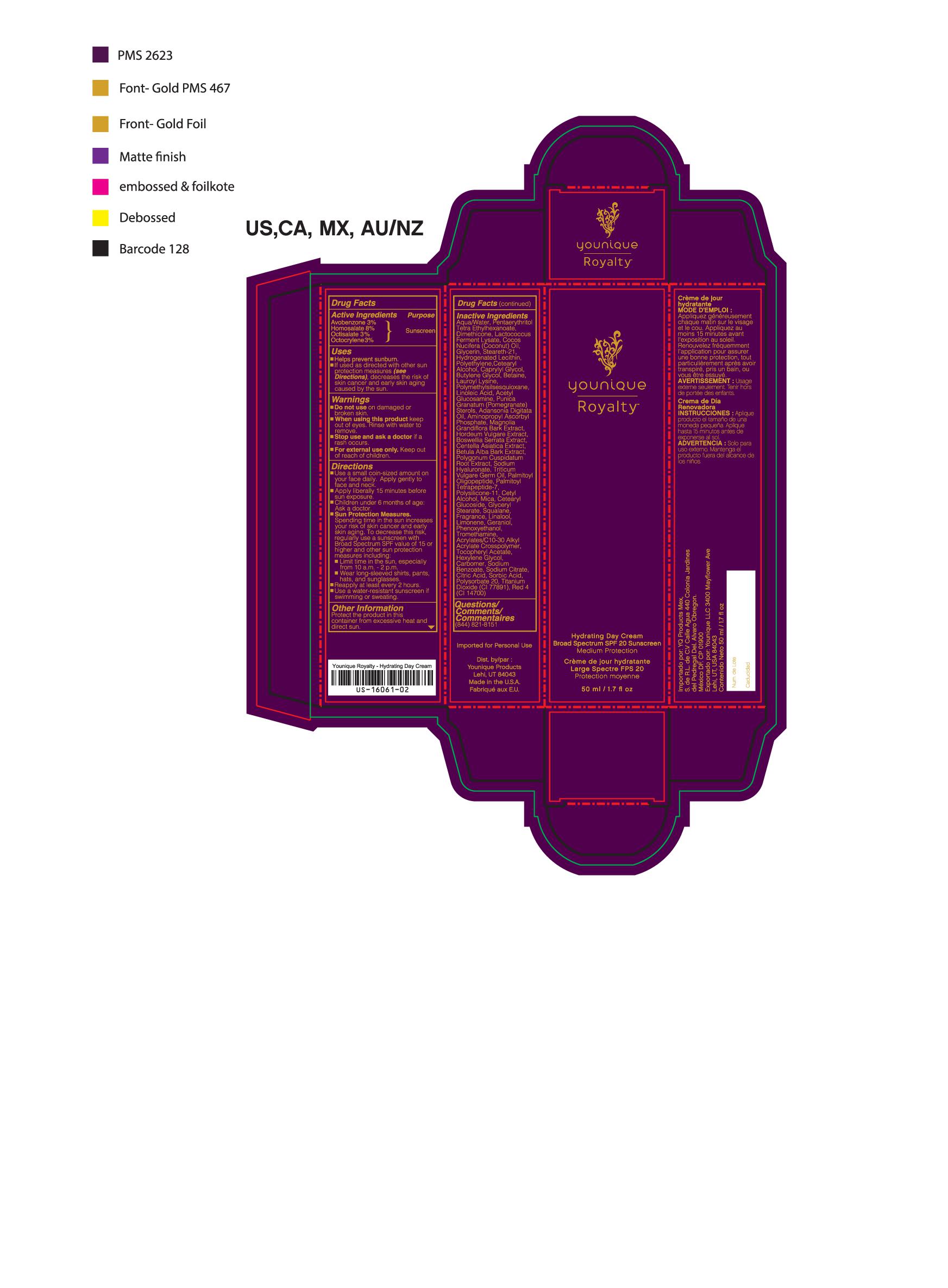
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
YOUNIQUE ROYALTY HYDRATING DAY CREAM SPF 20
suntan gels, creams, and liquids lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NHRIC:71182-3550 Route of Administration CUTANEOUS Other Ingredients Ingredient Kind Ingredient Name Quantity INGR OCTISALATE (UNII: 4X49Y0596W) 1.5 mL in 1 mL INGR OCTOCRYLENE (UNII: 5A68WGF6WM) 1.5 mL in 1 mL INGR HOMOSALATE (UNII: V06SV4M95S) 4 mL in 1 mL INGR N-ACETYLGLUCOSAMINE (UNII: V956696549) INGR AVOBENZONE (UNII: G63QQF2NOX) 1.5 mL in 1 mL INGR BUTYLENE GLYCOL (UNII: 3XUS85K0RA) INGR DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) INGR MICA (UNII: V8A1AW0880) INGR CAPRYLYL GLYCOL (UNII: 00YIU5438U) INGR GLYCERYL STEARATE SE (UNII: FCZ5MH785I) INGR SQUALANE (UNII: GW89575KF9) INGR PENTAERYTHRITYL TETRAETHYLHEXANOATE (UNII: XJ7052W897) INGR LOW DENSITY POLYETHYLENE (UNII: J245LN42AI) INGR ADANSONIA DIGITATA SEED OIL (UNII: 77MKL7AR5I) INGR HEXYLENE GLYCOL (UNII: KEH0A3F75J) INGR LINALOOL, (-)- (UNII: 3U21E3V8I2) INGR TROMETHAMINE (UNII: 023C2WHX2V) INGR LIMONENE, (+/-)- (UNII: 9MC3I34447) INGR COUMARIN (UNII: A4VZ22K1WT) INGR CARBOMER 940 (UNII: 4Q93RCW27E) INGR POMEGRANATE (UNII: 56687D1Z4D) INGR BARLEY (UNII: 5PWM7YLI7R) INGR CENTELLA ASIATICA (UNII: 7M867G6T1U) INGR HYALURONATE SODIUM (UNII: YSE9PPT4TH) INGR LACTOCOCCUS LACTIS (UNII: F1A0PSN10V) INGR LINOLEIC ACID (UNII: 9KJL21T0QJ) INGR WHEAT GERM OIL (UNII: 14C97E680P) INGR CETYL ALCOHOL (UNII: 936JST6JCN) INGR STEARETH-21 (UNII: 53J3F32P58) INGR HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) INGR DIMETHICONE 200 (UNII: RGS4T2AS00) INGR COCONUT OIL (UNII: Q9L0O73W7L) INGR CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) INGR BOSWELLIA SERRATA WHOLE (UNII: X7B7P649WQ) INGR BETULA PUBESCENS BARK (UNII: 3R504894L9) INGR POLYGONUM CUSPIDATUM ROOT (UNII: 7TRV45YZF7) INGR OLIGOPEPTIDE-10 (UNII: Q46328TRNK) INGR PALMITOYL TETRAPEPTIDE-7 (UNII: Q41S464P1R) INGR POLYSORBATE 20 (UNII: 7T1F30V5YH) INGR BETAINE (UNII: 3SCV180C9W) INGR LAUROYL LYSINE (UNII: 113171Q70B) INGR POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) INGR CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR ALPHA-TOCOPHERYLOXYACETIC ACID (UNII: JW7FJR3ZLY) INGR SODIUM BENZOATE (UNII: OJ245FE5EU) INGR GERANIOL (UNII: L837108USY) INGR .BETA.-CITRONELLOL, (+/-)- (UNII: 565OK72VNF) INGR CITRAL (UNII: T7EU0O9VPP) INGR FD&C RED NO. 4 (UNII: X3W0AM1JLX) INGR MAGNOLIA GRANDIFLORA BARK (UNII: J4XF5T6418) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR WATER (UNII: 059QF0KO0R) INGR AMINOPROPYL ASCORBYL PHOSPHATE (UNII: 290O2PQ83R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:71182-3550-1 1 in 1 CARTON 10/01/2016 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 10/01/2016 YOUNIQUE ROYALTY RENEWING DAY CREAM SPF 20
suntan gels, creams, and liquids lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NHRIC:71182-3440 Route of Administration CUTANEOUS Other Ingredients Ingredient Kind Ingredient Name Quantity INGR OCTISALATE (UNII: 4X49Y0596W) 1.5 mL in 1 mL INGR OCTOCRYLENE (UNII: 5A68WGF6WM) 1.5 mL in 1 mL INGR HOMOSALATE (UNII: V06SV4M95S) 4 mL in 1 mL INGR AVOBENZONE (UNII: G63QQF2NOX) 1.5 mL in 1 mL INGR BUTYLENE GLYCOL (UNII: 3XUS85K0RA) INGR DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) INGR SQUALANE (UNII: GW89575KF9) INGR N-ACETYLGLUCOSAMINE (UNII: V956696549) INGR ADANSONIA DIGITATA SEED OIL (UNII: 77MKL7AR5I) INGR LINALOOL, (-)- (UNII: 3U21E3V8I2) INGR TROMETHAMINE (UNII: 023C2WHX2V) INGR LIMONENE, (+/-)- (UNII: 9MC3I34447) INGR HEXYLENE GLYCOL (UNII: KEH0A3F75J) INGR COUMARIN (UNII: A4VZ22K1WT) INGR CARBOMER 940 (UNII: 4Q93RCW27E) INGR BARLEY (UNII: 5PWM7YLI7R) INGR CENTELLA ASIATICA (UNII: 7M867G6T1U) INGR HYALURONATE SODIUM (UNII: YSE9PPT4TH) INGR LACTOCOCCUS LACTIS (UNII: F1A0PSN10V) INGR CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) INGR POMEGRANATE (UNII: 56687D1Z4D) INGR CETYL ALCOHOL (UNII: 936JST6JCN) INGR STEARETH-21 (UNII: 53J3F32P58) INGR HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) INGR DIMETHICONE 200 (UNII: RGS4T2AS00) INGR LAURETH-12 (UNII: OAH19558U1) INGR POLYGONUM CUSPIDATUM ROOT (UNII: 7TRV45YZF7) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) INGR BOSWELLIA SERRATA WHOLE (UNII: X7B7P649WQ) INGR BETULA PUBESCENS BARK (UNII: 3R504894L9) INGR WHEAT GERM OIL (UNII: 14C97E680P) INGR OLIGOPEPTIDE-10 (UNII: Q46328TRNK) INGR PALMITOYL TETRAPEPTIDE-7 (UNII: Q41S464P1R) INGR POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR ALPHA-TOCOPHERYLOXYACETIC ACID (UNII: JW7FJR3ZLY) INGR SODIUM BENZOATE (UNII: OJ245FE5EU) INGR POLYSORBATE 20 (UNII: 7T1F30V5YH) INGR GERANIOL (UNII: L837108USY) INGR .BETA.-CITRONELLOL, (+/-)- (UNII: 565OK72VNF) INGR CITRAL (UNII: T7EU0O9VPP) INGR GLUTAMIC ACID (UNII: 3KX376GY7L) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR WATER (UNII: 059QF0KO0R) INGR AMINOPROPYL ASCORBYL PHOSPHATE (UNII: 290O2PQ83R) INGR MAGNOLIA GRANDIFLORA BARK (UNII: J4XF5T6418) INGR PROPANEDIOL (UNII: 5965N8W85T) INGR CYCLOMETHICONE 4 (UNII: CZ227117JE) INGR SALIX ALBA BARK (UNII: 205MXS71H7) INGR MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:71182-3440-1 1 in 1 CARTON 10/01/2016 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 10/01/2016 Labeler - Younique LLC (034555625) Registrant - Younique LLC (034555625) Establishment Name Address ID/FEI Business Operations Dermaceutical Laboratories LLC 078457159 manufacture(71182-3550, 71182-3440)