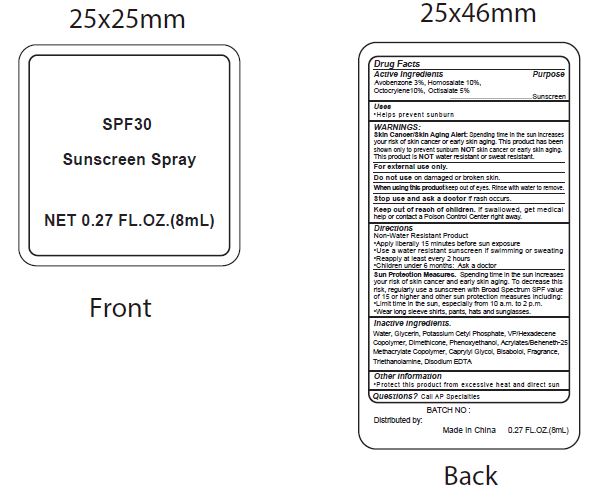
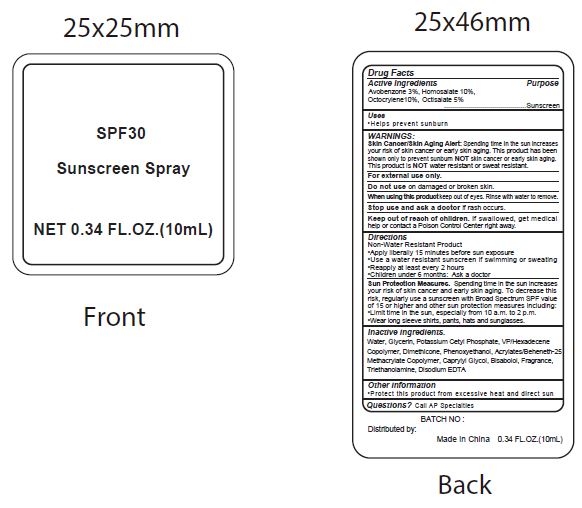
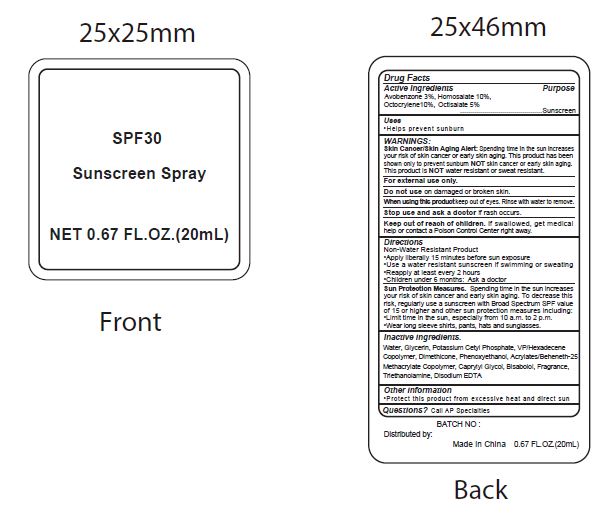
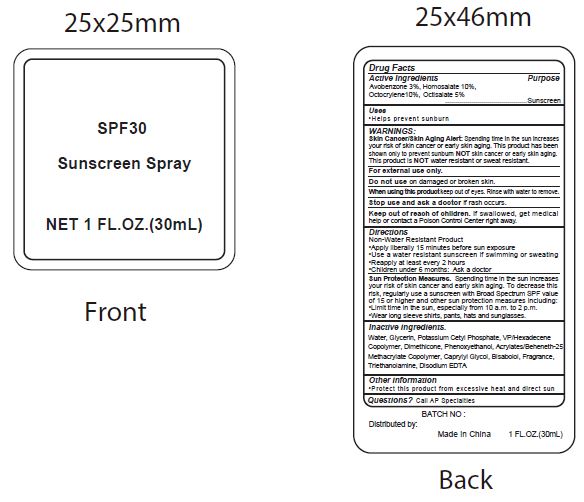
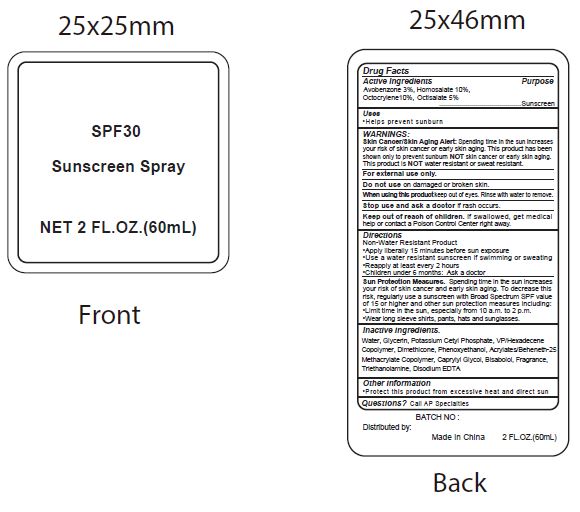
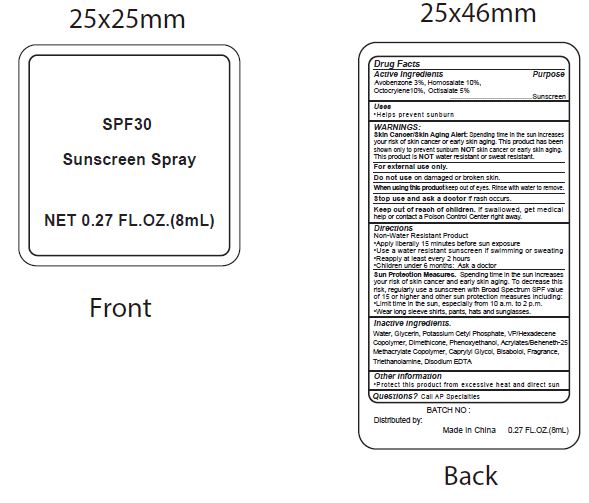
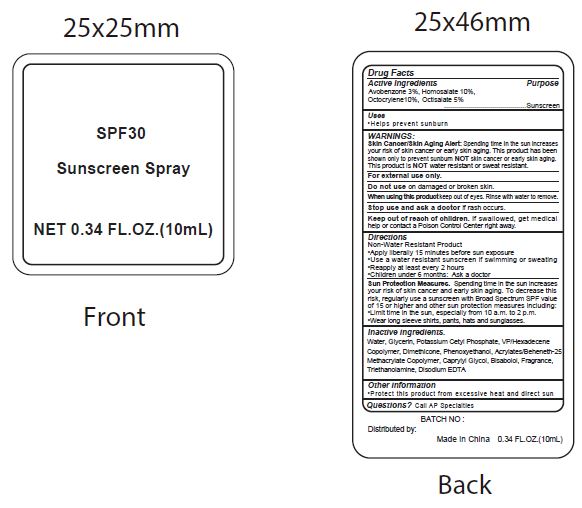
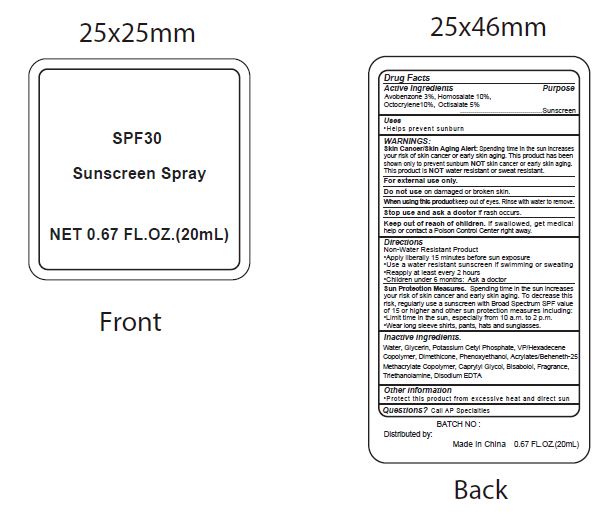
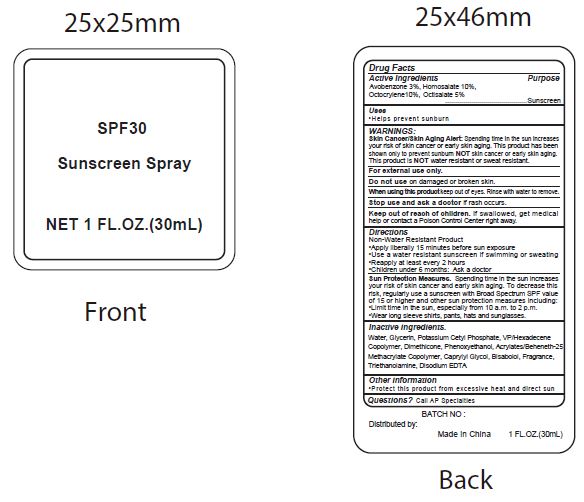
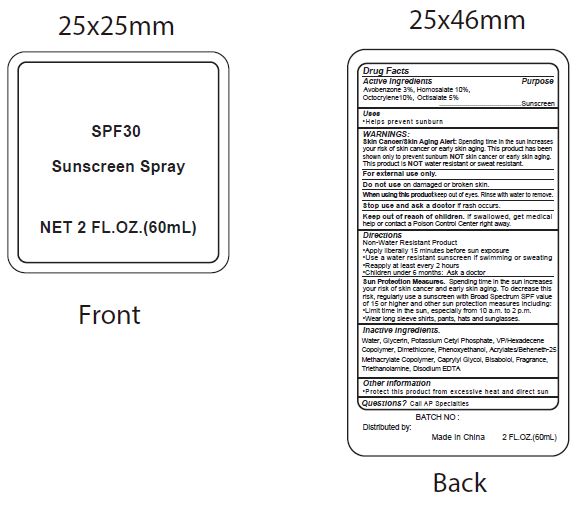
Label: SPF30 SUNSCREEN- avobenzone, homosalate, octocrylene, ocitsalate spray
-
NDC Code(s):
70412-238-05,
70412-238-08,
70412-238-10,
70412-238-20, view more70412-238-30, 70412-238-60
- Packager: Zhejiang Ayan Biotech Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
-
WARNINGS:
Spending time in the sun increases your risk of skin cnacer or early skin aging. this product has been shown only to prevent sunburn skin cancer or early skin aging. this product is water resistant or sweat resistant. Skin Cancer/Skin Aging Alert:NOTNOT
For external use only.
Do not use on damaged or broken skin.
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs.
- Keep out of reach of children.
- Directions
- inactive ingredients.
- Label
-
INGREDIENTS AND APPEARANCE
SPF30 SUNSCREEN
avobenzone, homosalate, octocrylene, ocitsalate sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70412-238 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 100 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 100 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) DIMETHICONE (UNII: 92RU3N3Y1O) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PHENOXYETHANOL (UNII: HIE492ZZ3T) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) VINYLPYRROLIDONE/HEXADECENE COPOLYMER (UNII: KFR5QEN0N9) LEVOMENOL (UNII: 24WE03BX2T) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) TROLAMINE (UNII: 9O3K93S3TK) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) COCONUT (UNII: 3RT3536DHY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70412-238-08 8 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2018 2 NDC:70412-238-20 20 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2018 3 NDC:70412-238-60 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2018 4 NDC:70412-238-30 30 mL in 1 BOTTLE; Type 1: Convenience Kit of Co-Package 06/01/2018 5 NDC:70412-238-10 10 mL in 1 PACKAGE; Type 0: Not a Combination Product 06/01/2018 6 NDC:70412-238-05 5 mL in 1 TUBE; Type 0: Not a Combination Product 06/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 06/01/2018 Labeler - Zhejiang Ayan Biotech Co., Ltd. (544377996) Establishment Name Address ID/FEI Business Operations Zhejiang Ayan Biotech Co., Ltd. 544377996 manufacture(70412-238)