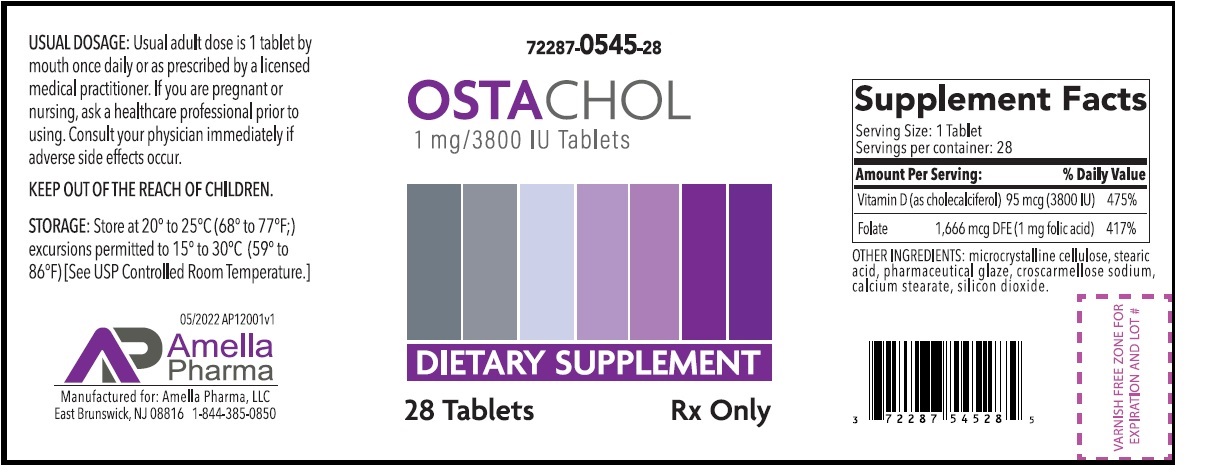
Label: OSTACHOL- vitamin d, folic acid tablet
- NHRIC Code(s): 72287-545-28
- Packager: Amella Pharma LLC
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated June 16, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Rx Only
OSTACHOL Tablets Dietary Supplement
Dispensed by Prescription**
Supplement Facts Serving Size:1 Tablet Servings per container: 28 Amount Per Serving: % Daily Value Vitamin D (as cholecalciferol) 95 mcg (3800 IU) 475% Folate 1,666 mcg DFE (1 mg folic acid) 417% OTHER INGREDIENTS: microcrystalline cellulose, stearic acid, pharmaceutical glaze, croscarmellose sodium, calcium stearate, silicon dioxide.
STATEMENT OF IDENTITY
OSTACHOL Tablets is an orally administered prescription folate product for the dietary management of patients with unique nutritional needs requiring increased folate levels and Vitamin D supplementation due to Vitamin D deficiency and other nutritional supplementation. OSTACHOL should be administered under the supervision of a licensed medical practitioner.OSTACHOL tablets are supplied as a light yellow tablet with “A1” imprinted on one side.
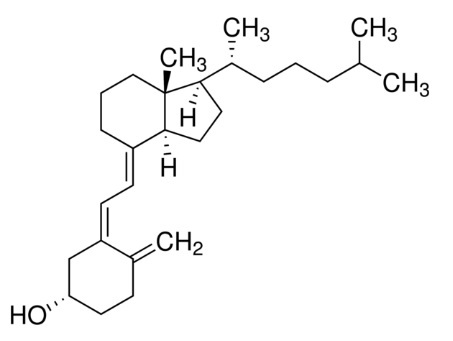
Vitamin D3 (cholecalciferol) is a white, crystalline powder, very soluble in water, with the following structural formula:
Each tablet contains:
Folic Acid............................................... 1,666 mcg DFE (1 mg)
Vitamin D3 (Cholecalciferol).......................... 95 mcg (3800IU)Each tablet contains the following inactive ingredients: microcrystalline cellulose, stearic acid, pharmaceutical glaze, croscarmellose sodium, calcium stearate, and silicon dioxide.
The in vivo synthesis of the major biologically active metabolites of vitamin D occurs in two steps. The first hydroxylation of ergocalciferol takes place in the liver (to 25-hydroxyvitamin D) and the second in the kidneys (to 1,25-dihydroxyvitamin D). Vitamin D metabolites promote the active absorption of calcium and phosphorus by the small intestine, thus elevating serum calcium and phosphate levels sufficiently to permit bone mineralization. Vitamin D metabolites also mobilize calcium and phosphate from bone and probably increase the reabsorption of calcium and perhaps also of phosphate by the renal tubules.
There is a time lag of 10 to 24 hours between the administration of vitamin D and the initiation of its action in the body due to the necessity of synthesis of the active metabolites in the liver and kidneys. Parathyroid hormone is responsible for the regulation of this metabolism in the kidneys.
-
HEALTH CLAIM:
OSTACHOL is used for dietary management of patients with unique nutritional needs requiring increased folate levels, Vitamin D deficiency or are in need of Vitamin D supplementation and other nutritional supplementation.
OSTACHOL can be taken by women of childbearing age, pregnant women, and lactating and nonlactating mothers.
-
PRECAUTIONS
CONTRAINDICATIONS:
This product is contraindicated in patients with known hypersensitivity to any of the ingredients.
PRECAUTIONS:
KEEP OUT OF THE REACH OF CHILDREN.
Tell your doctor if you have: kidney problems, thyroid disease. This medication should be used as directed during pregnancy or while breast-feeding. Consult your doctor about the risks and benefits.
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress.
- ADVERSE REACTIONS
- DOSAGE AND ADMINISTRATION:
-
HOW SUPPLIED:
OSTACHOL tablets are supplied as a light yellow tablet with “A1” imprinted on one side.
OSTACHOL Tablets is dispensed in child-resistant bottles as the following:
72287-545-28* 28ct bottleStore at 20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F) [See USP Controlled Room Temperature.]
Protect from heat, light and moisture.
Tamper Evident: Do not use if seal is broken or missing.
Manufactured for:
Amella Pharma, LLC
E Brunswick, NJ 08816 1-844-385-0850Issued: 05/2022 AP12001v1
*Amella Pharma does not represent these product codes to be National Drug Codes (NDC). Product codes are formatted according to standard industry practice, to meet the formatting requirement by pedigree reporting and supply-chain control including pharmacies.
** This product is a prescription-folate with or without other dietary ingredients that – due to increased folate levels (AUG 2 1973 FR 20750), requires an Rx on the label because of increased risk associated with masking of B12 deficiency (pernicious anemia). Based on our assessment of the risk of obscuring pernicious anemia, this product requires licensed medical supervision, an Rx status, and a National Drug Code (NDC) – or similarly-formatted product code, as required by pedigree reporting requirements and supply-chain control as well as – in some cases, for insurance-reimbursement applications.
All prescriptions using this product shall be pursuant to state statutes as applicable. This is not an Orange Book product. This product may be administered only under a physician’s supervision. There are no implied or explicit claims on therapeutic equivalence. - Packaging
-
INGREDIENTS AND APPEARANCE
OSTACHOL
vitamin d, folic acid tabletProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:72287-545 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 0.095 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STEARIC ACID (UNII: 4ELV7Z65AP) SHELLAC (UNII: 46N107B71O) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) CALCIUM STEARATE (UNII: 776XM7047L) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:72287-545-28 28 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 06/01/2022 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color shape size (solid drugs) 11 mm scoring 1 imprint Labeler - Amella Pharma LLC (081189492)