Label: PLERIXAFOR injection
- NDC Code(s): 70771-1776-1
- Packager: Zydus Lifesciences Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
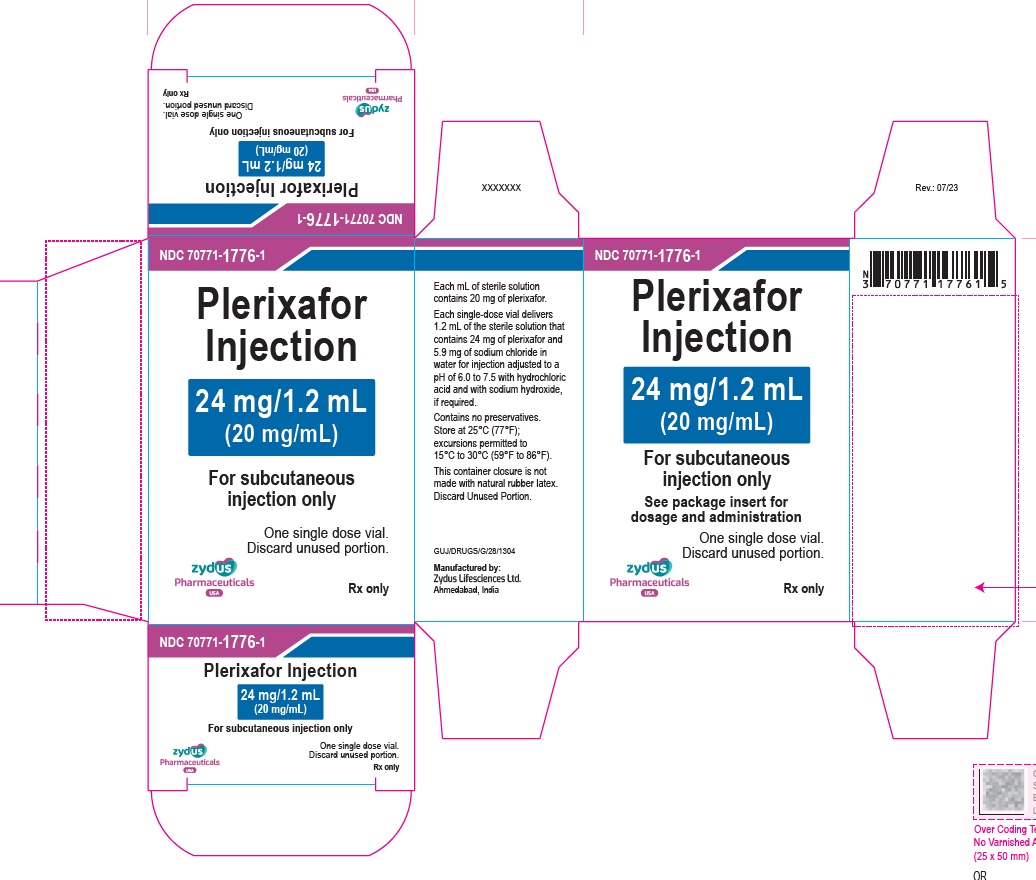
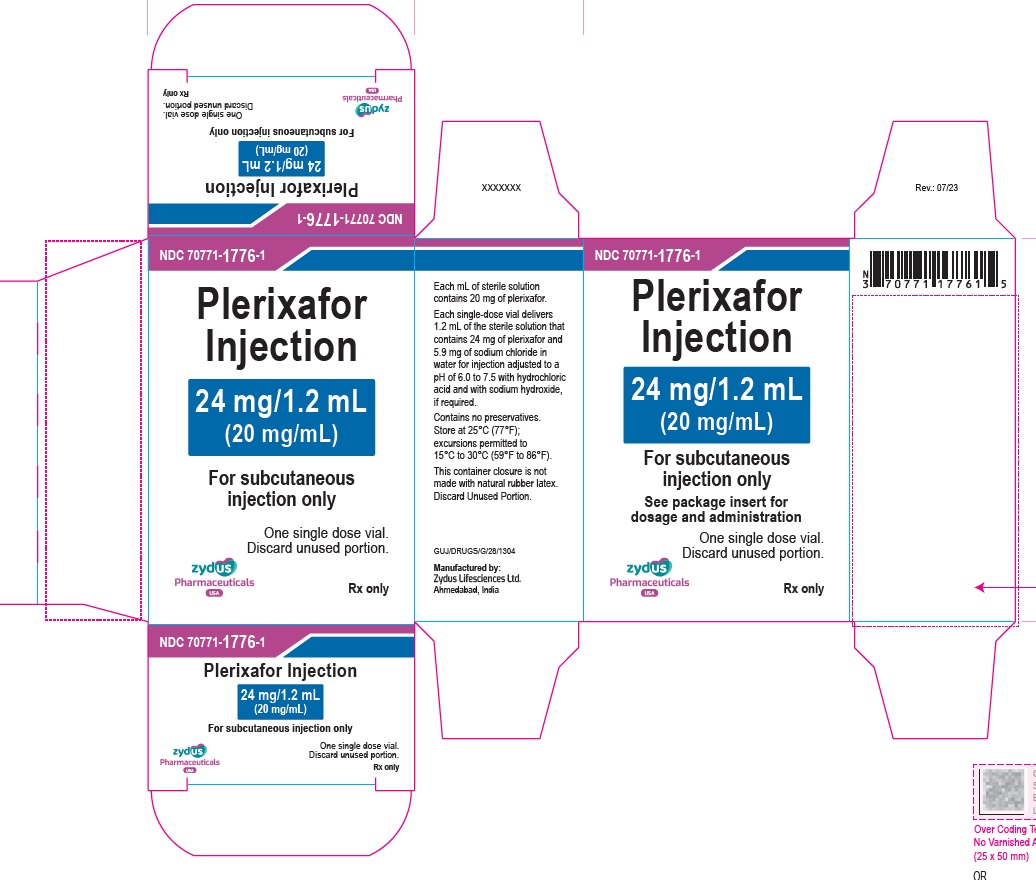
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Plerixafor Injection – 1.2 mL vial label
24 mg/1.2 mL
(20 mg/mL)
For subcutaneous injection only
For single-dose only
Rx only

Carton contains one vial of
Plerixafor Injection
24 mg/1.2 mL
(20 mg/mL)
For subcutaneous injection only
See package insert for dosage and administration
For single-dose only
Rx only
-
INGREDIENTS AND APPEARANCE
PLERIXAFOR
plerixafor injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1776 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PLERIXAFOR (UNII: S915P5499N) (PLERIXAFOR - UNII:S915P5499N) PLERIXAFOR 24 mg in 1.2 mL Inactive Ingredients Ingredient Name Strength HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM CHLORIDE (UNII: 451W47IQ8X) 5.9 mg in 1.2 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1776-1 1 in 1 CARTON 07/28/2023 1 1.2 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA208980 07/28/2023 Labeler - Zydus Lifesciences Limited (918596198) Establishment Name Address ID/FEI Business Operations Zydus Lifesciences Limited 650348852 MANUFACTURE(70771-1776) , ANALYSIS(70771-1776)