Label: CELONIA SIGNATURE BIO EYE- niacinamide, adenosine cream
- NDC Code(s): 73655-040-01, 73655-040-02
- Packager: Celino Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated March 10, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Inactive ingredients:
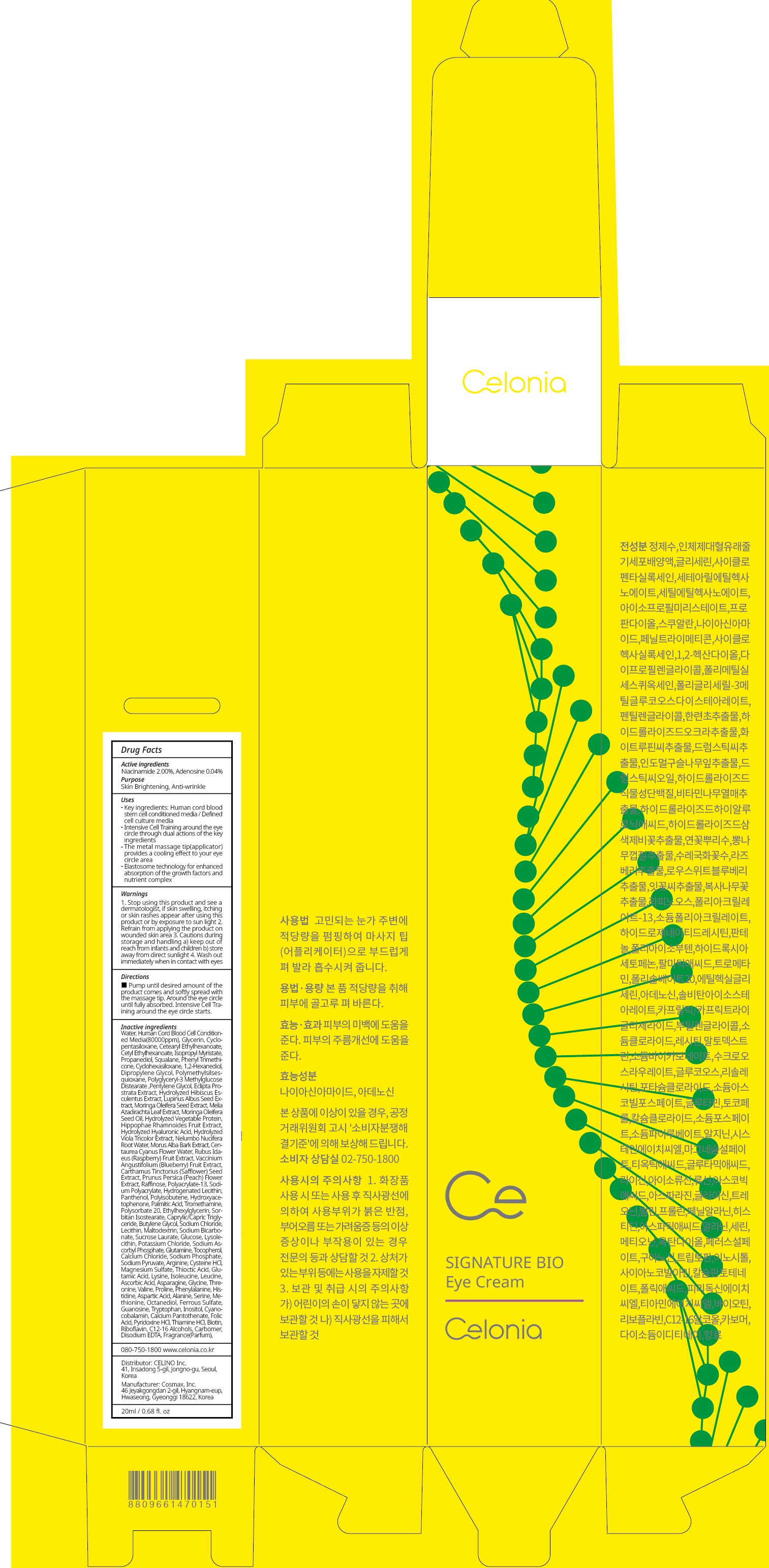
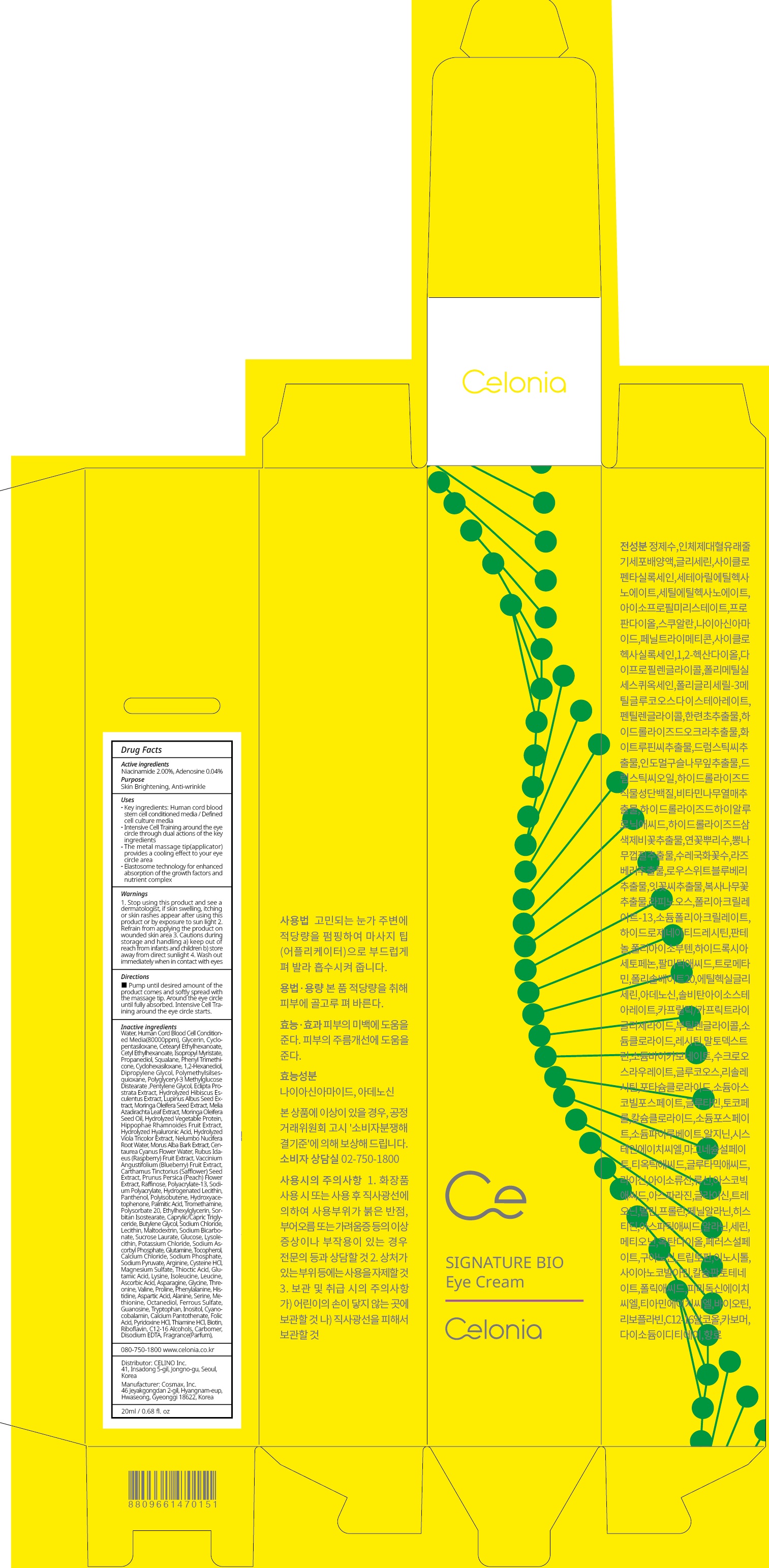
Water,Human Cord Blood Cell Conditioned Media(80000ppm),Glycerin,Cyclopentasiloxane,Cetearyl Ethylhexanoate,Cetyl Ethylhexanoate,Isopropyl Myristate,Propanediol,Squalane,Phenyl Trimethicone,Cyclohexasiloxane,1,2-Hexanediol,Dipropylene Glycol,Polymethylsilsesquioxane,Polyglyceryl-3 Methylglucose Distearate,Pentylene Glycol,Eclipta Prostrata Extract,Hydrolyzed Hibiscus Esculentus Extract,Lupinus Albus Seed Extract,Moringa Oleifera Seed Extract,Melia Azadirachta Leaf Extract,Moringa Oleifera Seed Oil,Hydrolyzed Vegetable Protein,Hippophae Rhamnoides Fruit Extract,Hydrolyzed Hyaluronic Acid,Hydrolyzed Viola Tricolor Extract,Nelumbo Nucifera Root Water,Morus Alba Bark Extract,Centaurea Cyanus Flower Water,Rubus Idaeus (Raspberry) Fruit Extract,Vaccinium Angustifolium (Blueberry) Fruit Extract,Carthamus Tinctorius (Safflower) Seed Extract,Prunus Persica (Peach) Flower Extract,Raffinose,Polyacrylate-13,Sodium Polyacrylate,Hydrogenated Lecithin,Panthenol,Polyisobutene,Hydroxyacetophenone,Palmitic Acid,Tromethamine,Polysorbate 20,Ethylhexylglycerin,Sorbitan Isostearate,Caprylic/Capric Triglyceride,Butylene Glycol,Sodium Chloride,Lecithin,Maltodextrin,Sodium Bicarbonate,Sucrose Laurate,Glucose,Lysolecithin,Potassium Chloride,Sodium Ascorbyl Phosphate,Glutamine,Tocopherol,Calcium Chloride,Sodium Phosphate,Sodium Pyruvate,Arginine,Cysteine HCl,Magnesium Sulfate,Thioctic Acid,Glutamic Acid,Lysine,Isoleucine,Leucine,Ascorbic Acid,Asparagine,Glycine,Threonine,Valine,Proline,Phenylalanine,Histidine,Aspartic Acid,Alanine,Serine,Methionine,Octanediol,Ferrous Sulfate,Guanosine,Tryptophan,Inositol,Cyanocobalamin,Calcium Pantothenate,Folic Acid,Pyridoxine HCl,Thiamine HCl,Biotin,Riboflavin,C12-16 Alcohols,Carbomer,Disodium EDTA,Fragrance(Parfum)
- PURPOSE
-
WARNINGS
Warnings:
1. Stop using this product and see a dermatologist, if skin swelling, itching or skin rashes appear after using this product or by exposure to sun light
2. Refrain from applying the product on wounded skin area
3. Cautions during storage and handling a) keep out of reach from infants and children b) store away from direct sunlight
4. Wash out immediately when in contact with eyes
- KEEP OUT OF REACH OF CHILDREN
- QUESTIONS
-
Uses
Uses:
Key ingredients: Human cord blood stem cell conditioned media / Defined cell culture media
Intensive Cell Training around the eye circle through dual actions of the key ingredients
The metal massage tip(applicator) provides a cooling effect to your eye circle area
Elastosome technology for enhanced absorption of the growth factors and nutrient complex - Directions
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CELONIA SIGNATURE BIO EYE
niacinamide, adenosine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73655-040 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Niacinamide (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) Niacinamide 0.40 g in 20 mL Adenosine (UNII: K72T3FS567) (ADENOSINE - UNII:K72T3FS567) Adenosine 0.008 g in 20 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73655-040-02 1 in 1 CARTON 02/01/2020 1 NDC:73655-040-01 20 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 02/01/2020 Labeler - Celino Inc. (695738827) Registrant - Celino Inc. (695738827) Establishment Name Address ID/FEI Business Operations Cosmax, Inc. 689049693 manufacture(73655-040)