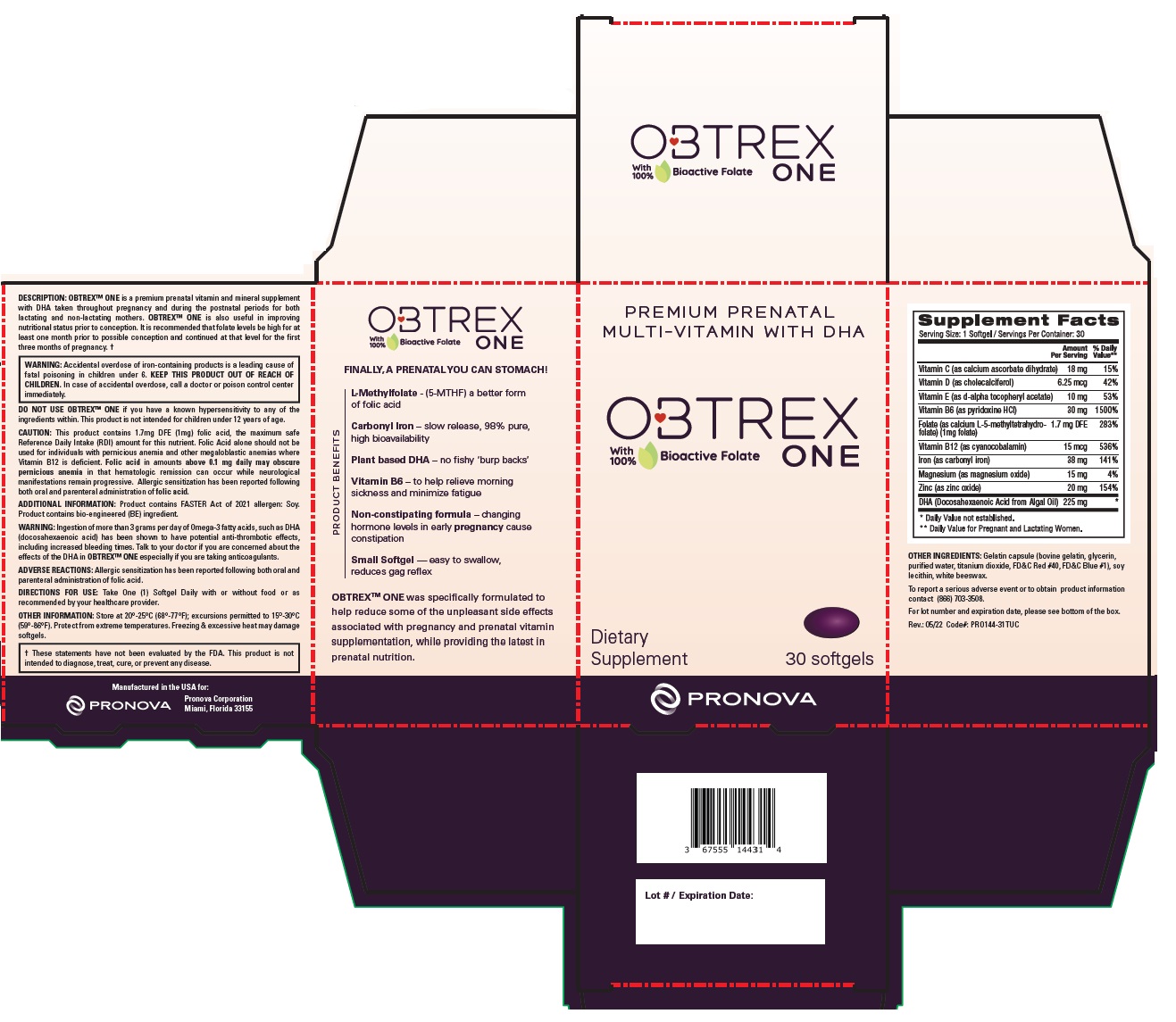
Label: OBTREX ONE- calcium ascorbate dihydrate, cholecalciferol, d-alpha tocopheryl acetate, pyridoxine hydrochloride, calcium l-5-methyltetrahydrofolate, cyanocobalamin, carbonyl iron, magnesium oxide, zinc oxide, docosahexaenoic acid capsule, gelatin coated
- NHRIC Code(s): 67555-144-31
- Packager: Pronova Corporation
- Category: DIETARY SUPPLEMENT
Drug Label Information
Updated June 5, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
STATEMENT OF IDENTITY
Supplement Facts Serving Size: 1 Softgel / Servings Per Container: 30 Amount
Per Serving
% Daily
Value**
Vitamin C (as calcium ascorbate dihydrate) 18 mg 15% Vitamin D (as cholecalciferol) 6.25 mcg 42% Vitamin E (as d-alpha tocopheryl acetate) 10 mg 53% Vitamin B6 (as pyridoxine HCl) 30 mg 1500% Folate (as L-5-methylfolate, calcium salt) - Metafolin® (1mg folate) 1.7 mg DFE 283% Vitamin B12 (as cyanocobalamin) 15 mcg 536% Iron (as carbonyl iron) 38 mg 141% Magnesium (as magnesium oxide) 15 mg 4% Zinc (as zinc oxide) 20 mg 154% DHA (Docosahexaenoic Acid from Algal Oil) 225 mg * * Daily Value not established. ** Daily Value for Pregnant and Lactating Women. OTHER INGREDIENTS: Gelatin capsule (bovine gelatin, glycerin, purified water, annatto, and sodium copper chlorophyllin), sunflower lecithin, white beeswax.
Metafolin® is a registered trademark of Merck KGaA, Darmstadt Germany.
To report a serious adverse event or to obtain product information contact (866) 703-3508.
For lot number and expiration date, please see bottom of the box.
Revised 01/2024 Code#: PRO144-31TUCDESCRIPTION: OBTREX™ ONE is a premium prenatal vitamin and mineral supplement with DHA taken throughout pregnancy and during the postnatal periods for both lactating and non-lactating mothers. OBTREX™ ONE is also useful in improving nutritional status prior to conception. It is recommended that folate levels be high for at least one month prior to possible conception and continued at that level for the first three months of pregnancy. †
-
WARNINGS
WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. KEEP THIS PRODUCT OUT OF REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately. DO NOT USE OBTREX™ ONE if you have a known hypersensitivity to any of the ingredients within. This product is not intended for children under 12 years of age.
WARNING: Ingestion of more than 3 grams per day of Omega-3 fatty acids, such as DHA (docosahexaenoic acid) has been shown to have potential anti-thrombotic effects, including increased bleeding times. Talk to your doctor if you are concerned about the effects of the DHA in OBTREX™ ONE especially if you are taking anticoagulants.
-
CAUTION:
This product contains 1.7mg DFE (1mg) folic acid, the maximum safe Reference Daily Intake (RDI) amount for this nutrient. Folic Acid alone should not be used for individuals with pernicious anemia and other megaloblastic anemias where Vitamin B12 is deficient. Folic acid in amounts above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations remain progressive. Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
ADDITIONAL INFORMATION: Potential discoloration and/or darkening on capsules or capsule seams may occur immediately or overtime, due to the Vitamin C and Iron. Does not affect product efficacy.
ADVERSE REACTIONS: Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
- DIRECTIONS FOR USE:
- OTHER INFORMATION:
-
HEALTH CLAIM
Dietary Supplement
PREMIUM PRENATAL MULTI-VITAMIN WITH DHA
with 100% Bioactive Folate
NO ARTIFICIAL COLORANTS OR DYES
FINALLY, A PRENATAL YOU CAN STOMACH!
PRODUCT BENEFITS
Metafolin® - increases plasma folate more effectively than folic acid
Carbonyl Iron – slow release, 98% pure, high bioavailability
Plant based DHA – no fishy ‘burp backs’
Vitamin B6 – to help relieve morning sickness and minimize fatigue
Non-constipating formula – changing hormone levels in early pregnancy cause constipation
Small, All-in-One Softgel — easy to swallow, well-tolerated
OBTREX™ ONE was specifically formulated to help reduce some of the unpleasant side effects associated with pregnancy and prenatal vitamin supplementation, while providing the latest in prenatal nutrition.
Manufactured in the USA for:
Pronova Corporation
Miami, Florida 33155 - Packaging
-
INGREDIENTS AND APPEARANCE
OBTREX ONE
calcium ascorbate dihydrate, cholecalciferol, d-alpha tocopheryl acetate, pyridoxine hydrochloride, calcium l-5-methyltetrahydrofolate, cyanocobalamin, carbonyl iron, magnesium oxide, zinc oxide, docosahexaenoic acid capsule, gelatin coatedProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:67555-144 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM ASCORBATE (UNII: 183E4W213W) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 18 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 6.25 ug .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) (.ALPHA.-TOCOPHEROL, D- - UNII:N9PR3490H9) .ALPHA.-TOCOPHEROL, D- 10 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 30 mg LEVOMEFOLATE CALCIUM (UNII: A9R10K3F2F) (LEVOMEFOLIC ACID - UNII:8S95DH25XC) LEVOMEFOLATE CALCIUM 1 mg CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 15 ug IRON (UNII: E1UOL152H7) (IRON - UNII:E1UOL152H7) IRON 38 mg MAGNESIUM OXIDE (UNII: 3A3U0GI71G) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM OXIDE 15 mg ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 20 mg DOCONEXENT (UNII: ZAD9OKH9JC) (DOCONEXENT - UNII:ZAD9OKH9JC) DOCONEXENT 225 mg Inactive Ingredients Ingredient Name Strength GELATIN TYPE B BOVINE (230 BLOOM) (UNII: WIL1404U79) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) ANNATTO (UNII: 6PQP1V1B6O) SODIUM COPPER CHLOROPHYLLIN (UNII: 1D276TYV9O) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) WHITE WAX (UNII: 7G1J5DA97F) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:67555-144-31 3 in 1 BOX 1 10 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 03/30/2023 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color shape size (solid drugs) 14 mm scoring 1 Labeler - Pronova Corporation (111421496)