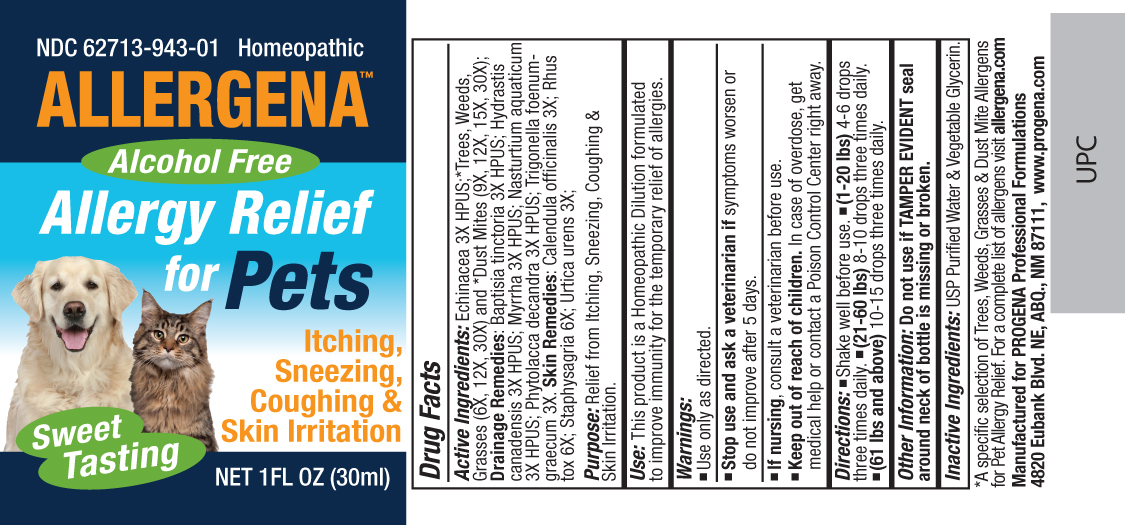
Label: ALLERGENA FOR PETS liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 62713-943-01 - Packager: Meditrend, Inc. DBA Progena Professional Formulations
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated October 16, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Active Ingredients:Echinacea 3X HPUS;*Trees, Weeds, Grasses (6X, 12X, 30X) and *Dust Mites (9X, 12X, 15X, 30X);Drainage Remedies: Baptisia tinctoria 3X HPUS; Hydrastis canadensis 3X HPUS; Myrrha 3X HPUS; Nasturtium aquaticum 3X HPUS; Phytolacca decandra 3X HPUS; Trigonella foenum-graecum 3X.Skin Remedies:Calendula officinalis 3X; Rhus tox 6X; Staphysagria 6X; Urtica urens 3X;
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ALLERGENA FOR PETS
allergena for pets liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62713-943 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ECHINACEA, UNSPECIFIED (UNII: 4N9P6CC1DX) (ECHINACEA, UNSPECIFIED - UNII:4N9P6CC1DX) ECHINACEA, UNSPECIFIED 3 [hp_X] in 1 mL BETULA PAPYRIFERA POLLEN (UNII: 3538FNV8AY) (BETULA PAPYRIFERA POLLEN - UNII:3538FNV8AY) BETULA PAPYRIFERA POLLEN 30 [hp_X] in 1 mL JUNIPERUS ASHEI POLLEN (UNII: 544F8MEY0Y) (JUNIPERUS ASHEI POLLEN - UNII:544F8MEY0Y) JUNIPERUS ASHEI POLLEN 30 [hp_X] in 1 mL QUERCUS RUBRA POLLEN (UNII: SVW19ET93C) (QUERCUS RUBRA POLLEN - UNII:SVW19ET93C) QUERCUS RUBRA POLLEN 30 [hp_X] in 1 mL ARTEMISIA VULGARIS POLLEN (UNII: ANT994T71D) (ARTEMISIA VULGARIS POLLEN - UNII:ANT994T71D) ARTEMISIA VULGARIS POLLEN 30 [hp_X] in 1 mL AMARANTHUS RETROFLEXUS POLLEN (UNII: 73B14PX5FW) (AMARANTHUS RETROFLEXUS POLLEN - UNII:73B14PX5FW) AMARANTHUS RETROFLEXUS POLLEN 30 [hp_X] in 1 mL AMBROSIA ARTEMISIIFOLIA POLLEN (UNII: K20Y81ACO3) (AMBROSIA ARTEMISIIFOLIA POLLEN - UNII:K20Y81ACO3) AMBROSIA ARTEMISIIFOLIA POLLEN 30 [hp_X] in 1 mL SALSOLA TRAGUS POLLEN (UNII: V174354MDI) (SALSOLA TRAGUS POLLEN - UNII:V174354MDI) SALSOLA TRAGUS POLLEN 30 [hp_X] in 1 mL PASPALUM NOTATUM POLLEN (UNII: V003SHB7VK) (PASPALUM NOTATUM POLLEN - UNII:V003SHB7VK) PASPALUM NOTATUM POLLEN 30 [hp_X] in 1 mL CYNODON DACTYLON POLLEN (UNII: 175F461W10) (CYNODON DACTYLON POLLEN - UNII:175F461W10) CYNODON DACTYLON POLLEN 30 [hp_X] in 1 mL POA ANNUA POLLEN (UNII: 7U437HHU5C) (POA ANNUA POLLEN - UNII:7U437HHU5C) POA ANNUA POLLEN 30 [hp_X] in 1 mL LOLIUM PERENNE POLLEN (UNII: 4T81LB52R0) (LOLIUM PERENNE POLLEN - UNII:4T81LB52R0) LOLIUM PERENNE POLLEN 30 [hp_X] in 1 mL PHLEUM PRATENSE POLLEN (UNII: 65M88RW2EG) (PHLEUM PRATENSE POLLEN - UNII:65M88RW2EG) PHLEUM PRATENSE POLLEN 30 [hp_X] in 1 mL DERMATOPHAGOIDES PTERONYSSINUS (UNII: 57L1Z5378K) (DERMATOPHAGOIDES PTERONYSSINUS - UNII:57L1Z5378K) DERMATOPHAGOIDES PTERONYSSINUS 30 [hp_X] in 1 mL DERMATOPHAGOIDES FARINAE (UNII: PR9U2YPF3Q) (DERMATOPHAGOIDES FARINAE - UNII:PR9U2YPF3Q) DERMATOPHAGOIDES FARINAE 30 [hp_X] in 1 mL BAPTISIA TINCTORIA ROOT (UNII: 5EF0HWI5WU) (BAPTISIA TINCTORIA ROOT - UNII:5EF0HWI5WU) BAPTISIA TINCTORIA ROOT 3 [hp_X] in 1 mL GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 3 [hp_X] in 1 mL MYRRH (UNII: JC71GJ1F3L) (MYRRH - UNII:JC71GJ1F3L) MYRRH 3 [hp_X] in 1 mL NASTURTIUM OFFICINALE (UNII: YH89GMV676) (NASTURTIUM OFFICINALE - UNII:YH89GMV676) NASTURTIUM OFFICINALE 3 [hp_X] in 1 mL PHYTOLACCA AMERICANA ROOT (UNII: 11E6VI8VEG) (PHYTOLACCA AMERICANA ROOT - UNII:11E6VI8VEG) PHYTOLACCA AMERICANA ROOT 3 [hp_X] in 1 mL FENUGREEK LEAF (UNII: 487RI96K8Z) (FENUGREEK LEAF - UNII:487RI96K8Z) FENUGREEK LEAF 3 [hp_X] in 1 mL CALENDULA OFFICINALIS FLOWERING TOP (UNII: 18E7415PXQ) (CALENDULA OFFICINALIS FLOWERING TOP - UNII:18E7415PXQ) CALENDULA OFFICINALIS FLOWERING TOP 3 [hp_X] in 1 mL TOXICODENDRON PUBESCENS LEAF (UNII: 6IO182RP7A) (TOXICODENDRON PUBESCENS LEAF - UNII:6IO182RP7A) TOXICODENDRON PUBESCENS LEAF 6 [hp_X] in 1 mL DELPHINIUM STAPHISAGRIA SEED (UNII: 00543AP1JV) (DELPHINIUM STAPHISAGRIA SEED - UNII:00543AP1JV) DELPHINIUM STAPHISAGRIA SEED 6 [hp_X] in 1 mL URTICA URENS (UNII: IHN2NQ5OF9) (URTICA URENS - UNII:IHN2NQ5OF9) URTICA URENS 3 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62713-943-01 30 mL in 1 BOTTLE, DROPPER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/01/1987 Labeler - Meditrend, Inc. DBA Progena Professional Formulations (130104805)