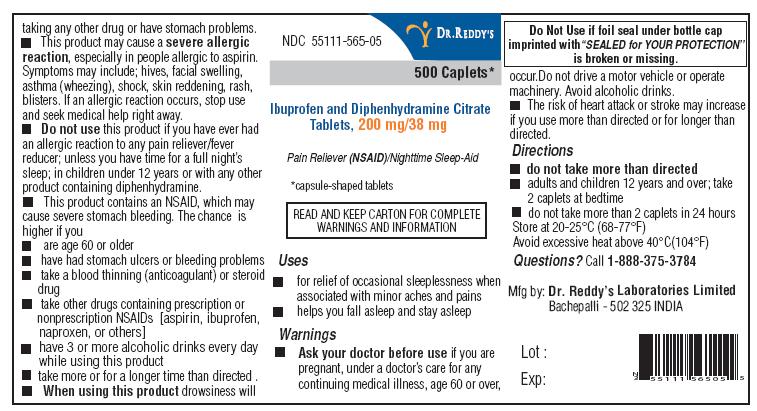
Label: IBUPROFEN AND DIPHENHYDRAMINE CITRATE tablet
-
NDC Code(s):
55111-565-05,
55111-565-14,
55111-565-18,
55111-565-30, view more55111-565-40, 55111-565-80, 55111-565-90, 55111-565-92
- Packager: Dr. Reddy's Laboratories Limited
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 5, 2018
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DRUG FACTS
- ACTIVE INGREDIENTS (IN EACH CAPLET)
- PURPOSES
- USES
-
WARNINGS
Allergy alert:
Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
- skin reddening
- rash
- blisters
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning:
This product contains an NSAID, which may cause stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing an NSAID [aspirin, ibuprofen, naproxen, or others]
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Do not use
- if you have ever had an allergic reaction to any other pain reliever/fever reducer
- unless you have time for a full night's sleep
- in children under 12 years of age
- right before or after heart surgery
- with any other product containing diphenhydramine, even one used on skin
- if you have sleeplessness without pain.
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have problems or serious side effects from taking pain relievers or fever reducers
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, kidney disease, or asthma
- you are taking a diuretic
- you have a breathing problem such as emphysema or chronic bronchitis
- you have glaucoma
- you have trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you are
- taking sedatives or tranquilizers, or any other sleep-aid
- under a doctor’s care for any continuing medical illness
- taking any other antihistamines
- taking aspirin for heart attack or stroke, because ibuprofen may decrease this benefit of aspirin
- taking any other drug
When using this product
- drowsiness will occur
- avoid alcoholic drinks
- do not drive a motor vehicle or operate machinery
- take with food or milk if stomach upset occurs
- the risk of heart attack or stroke may increase if you use more than directed or for longer than directed
Stop use and ask a doctor if
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- pain gets worse or lasts more than 10 days
- sleeplessness persists continuously for more than 2 weeks. Insomnia may be a symptom of a serious underlying medical illness.
- redness or swelling is present in the painful area
- any new symptoms appear
- DIRECTIONS
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- QUESTIONS?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
IBUPROFEN AND DIPHENHYDRAMINE CITRATE
ibuprofen and diphenhydramine citrate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55111-565 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Ibuprofen (UNII: WK2XYI10QM) (Ibuprofen - UNII:WK2XYI10QM) Ibuprofen 200 mg Diphenhydramine Citrate (UNII: 4OD433S209) (Diphenhydramine - UNII:8GTS82S83M) Diphenhydramine Citrate 38 mg Inactive Ingredients Ingredient Name Strength carnauba wax (UNII: R12CBM0EIZ) STARCH, CORN (UNII: O8232NY3SJ) Silicon Dioxide (UNII: ETJ7Z6XBU4) croscarmellose sodium (UNII: M28OL1HH48) FD&C blue no. 2 (UNII: L06K8R7DQK) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) hypromelloses (UNII: 3NXW29V3WO) polydextrose (UNII: VH2XOU12IE) polyethylene glycol 400 (UNII: B697894SGQ) sodium lauryl sulfate (UNII: 368GB5141J) stearic acid (UNII: 4ELV7Z65AP) titanium dioxide (UNII: 15FIX9V2JP) Product Characteristics Color BLUE (blue) Score no score Shape CAPSULE (slightly glossy smooth blue film coated) Size 15mm Flavor Imprint Code RDY;565 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55111-565-14 1 in 1 CARTON 01/31/2010 1 20 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:55111-565-30 1 in 1 CARTON 01/31/2010 2 30 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:55111-565-40 1 in 1 CARTON 01/31/2010 3 40 in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC:55111-565-80 1 in 1 CARTON 01/31/2010 4 80 in 1 BOTTLE; Type 0: Not a Combination Product 5 NDC:55111-565-90 1 in 1 CARTON 01/31/2010 5 90 in 1 BOTTLE; Type 0: Not a Combination Product 6 NDC:55111-565-18 1 in 1 CARTON 01/31/2010 6 180 in 1 BOTTLE; Type 0: Not a Combination Product 7 NDC:55111-565-05 1 in 1 CARTON 01/31/2010 7 500 in 1 BOTTLE; Type 0: Not a Combination Product 8 NDC:55111-565-92 2500 in 1 POUCH; Type 0: Not a Combination Product 01/31/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090619 01/31/2010 Labeler - Dr. Reddy's Laboratories Limited (650562841)