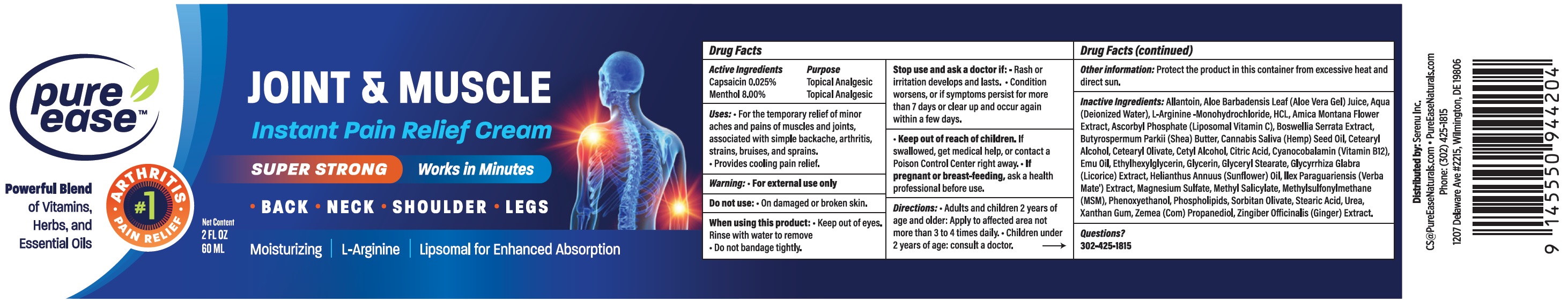
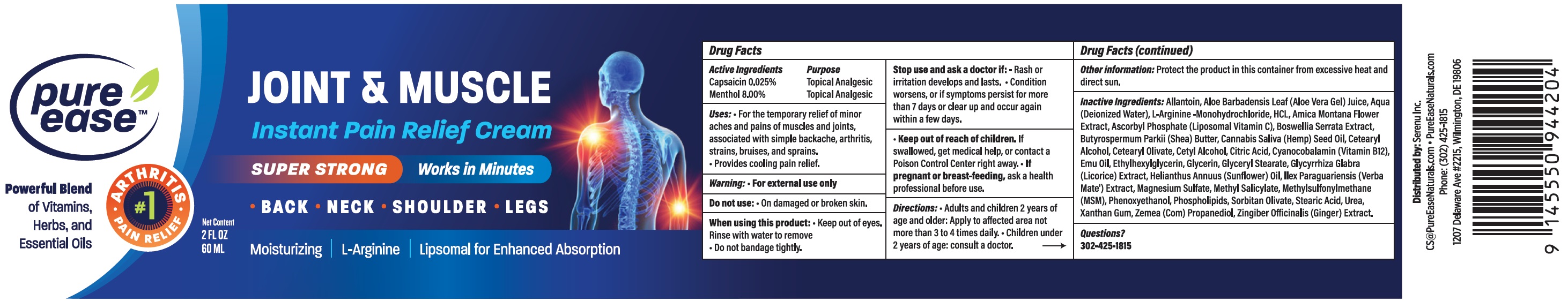
Label: PURE EASE JOINT MUSCLE LNSTANT PAIN RELIEF- capsaicin, menthol cream
- NDC Code(s): 83676-435-00
- Packager: SERENU, INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Uses:
-
Warning:
For external use only
Stop use and ask a doctor if:
- Rash or irritation develops and lasts.
- Condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days.
- Directions:
- Other information:
-
Inactive Ingredients:
Allantoin, Aloe Barbadnesis Leaf (Aloe Vera Gel) Juice, Aqua (Deionized Water), L-Arginine-Monohydrochloride, HCL, Amica Montana Flower Extract, Ascorbyl Phosphate (Liposomal Vitamin C), Boswellia Serrata Extract, Butyrospermum Parkii (Shea) Butter, Cannabis Saliva (Hemp) Seed Oil, Cetearyl Alcohol, Cetearyl Olivate, Cetyl Alcohol, Citric Acid, Cyanocobalamin (Vitamin B12), Emu Oil, Ethylhexylglycerin, Glycerin, Glyceryl Stearate, Glycyrrhiza Glabra (Licorice) Extract, Helianthus Annuus (Sunflower) Oil, Ilex Paraquariensis (Verba Mate') Extract, Magnesium Sulfate, Methylsulfonylmethane (MSM), Phenoxyethanol, Phospholipids, Sorbitan Olivate, Stearic Acid, Urea, Xanthan Gum, Zemea (Com) Propanediol, Zingiber Officinalis (Ginger) Extract.
- Questions?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
PURE EASE JOINT MUSCLE LNSTANT PAIN RELIEF
capsaicin, menthol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83676-435 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 0.25 mg in 1 mL MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 80 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALLANTOIN (UNII: 344S277G0Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) ARGININE HYDROCHLORIDE (UNII: F7LTH1E20Y) TALINEXOMER (UNII: AZC253V6FB) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) MAGNESIUM ASCORBYL PHOSPHATE (UNII: 0R822556M5) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) SHEA BUTTER (UNII: K49155WL9Y) HEMP (UNII: TD1MUT01Q7) CANNABIS SATIVA SEED OIL (UNII: 69VJ1LPN1S) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETEARYL OLIVATE (UNII: 58B69Q84JO) CETYL ALCOHOL (UNII: 936JST6JCN) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) CYANOCOBALAMIN (UNII: P6YC3EG204) EMU OIL (UNII: 344821WD61) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL 1-STEARATE (UNII: 258491E1RZ) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) HELIANTHUS ANNUUS FLOWERING TOP (UNII: BKJ0J3D1BP) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) METHYL SALICYLATE (UNII: LAV5U5022Y) DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) PHENOXYETHANOL (UNII: HIE492ZZ3T) SORBITAN OLIVATE (UNII: MDL271E3GR) STEARIC ACID (UNII: 4ELV7Z65AP) UREA (UNII: 8W8T17847W) XANTHAN GUM (UNII: TTV12P4NEE) CORN (UNII: 0N8672707O) GINGER (UNII: C5529G5JPQ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83676-435-00 60 mL in 1 JAR; Type 0: Not a Combination Product 11/27/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 11/27/2023 Labeler - SERENU, INC. (119079642)