Label: 3M CAVILON DURABLE BARRIER- dimethicone cream
- NDC Code(s): 17518-030-04, 17518-030-05, 17518-030-06
- Packager: Solventum US OpCo LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated April 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Uses:
- Directions:
- Storage Conditions:
- Usages :
-
Mode d'emploi :
Nettoyer délicatement la peau selon les besoins. Appliquer la Crème protectrice durable CavilonMC 3MMC avec modération afin de couvrir l'ensemble de la zone atteinte. Répéter au besoin. Si la peau est grasse au toucher, c'est qu'une trop grande quantité de crème a été appliquée.
Ingrédient actif (p/p) : Diméthicone à 1,3 %
-
Conditions d'entreposage :
Entreposer à une température de 15 °C à 30 °C (59 °F à 86 °F). Consulter l'étiquette ou le carton extérieur pour obtenir des renseignements complets sur les usages, le mode d'emploi, les avertissements ainsi que les précautions.
3M CANADA COMPANY
LA COMPAGNIE 3M CANADA
300 TARTAN DRIVE, LONDON, ON N5V 4M9
QUESTIONS? 1-800-364-3577
www.3M.ca
3M and Cavilon are trademarks of 3M. Used under license in Canada. 3M et Cavilon sont des marques de commerce de 3M, utilisées sous licence au Canada. © 2021, 3M. All rights reserved. Tous droits réservés. 34-8727-0667-5
-
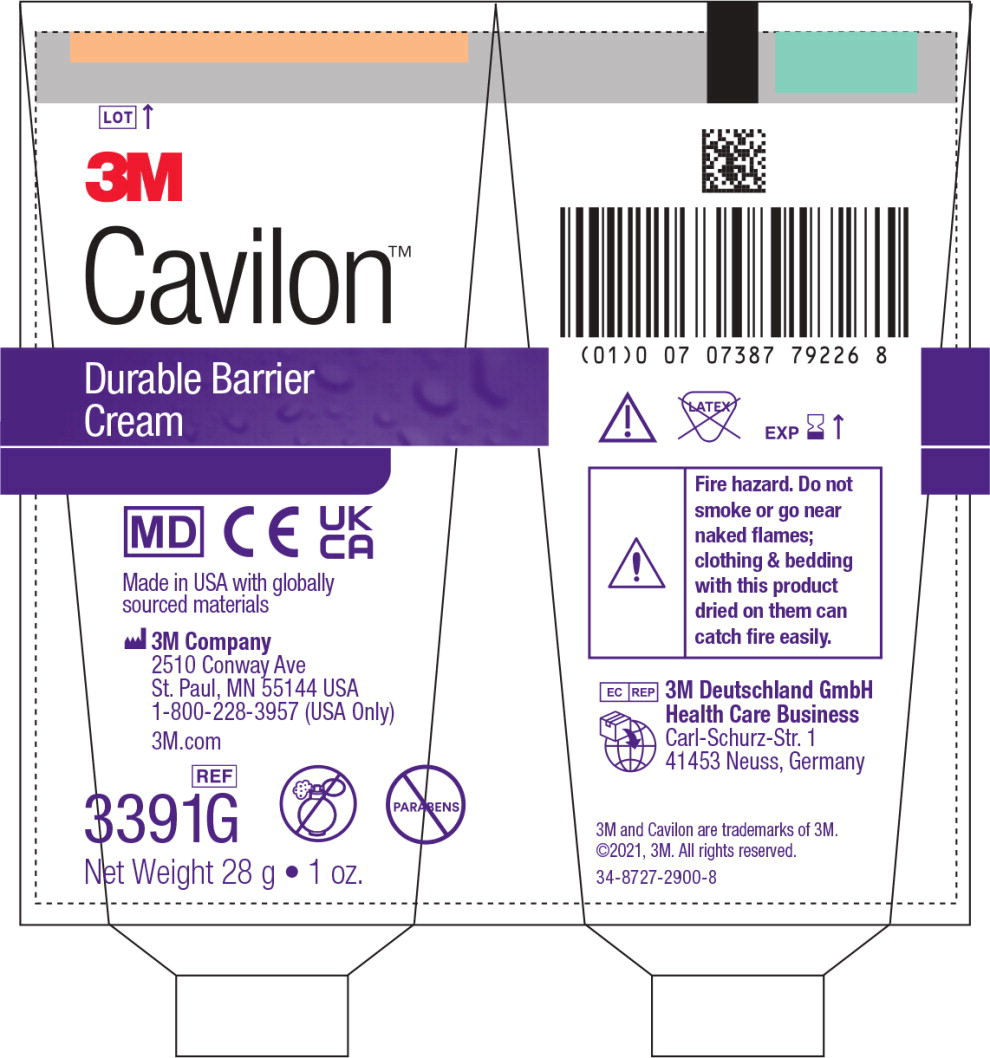
Principal Display Panel – CA Tube Label (28g)
LOT
3M
DIN 02455838
Cavilon™/MC
Durable Barrier Cream
Crème protectrice durableDimethicone Cream NF Skin Protectant 1.3% (w/w)
Protecteur cutané à la diméthicone NF 1,3 %(p/p)
Protects from incontinence
- Resists wash-off •Concentrated
- Allows tape to stick
- Moisturizes and conditions dry skin
- Fragrance free •Parabens free
Protège contre les effets de l'incontinence
- Résiste au rinçage • Concentrée
- Permet au ruban adhésif d'adhérer
- Hydrate et revitalise la peau sèche
- Non parfumée • Sans parabènes
REF
3991C 28g
- Principal Display Panel – EU Tube Label (28g)
- Principal Display Panel – UKCA Tube Label (28g)
-
INGREDIENTS AND APPEARANCE
3M CAVILON DURABLE BARRIER
dimethicone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17518-030 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Dimethicone (UNII: 92RU3N3Y1O) (Dimethicone - UNII:92RU3N3Y1O) Dimethicone 13000 mg in 1 L Inactive Ingredients Ingredient Name Strength Benzoic Acid (UNII: 8SKN0B0MIM) Coconut Oil (UNII: Q9L0O73W7L) Dehydroacetic Acid (UNII: 2KAG279R6R) Diisooctyl Adipate (UNII: 2TLQ9O0CWB) Glycerin (UNII: PDC6A3C0OX) Isopropyl Palmitate (UNII: 8CRQ2TH63M) Magnesium Sulfate Heptahydrate (UNII: SK47B8698T) Mineral Oil (UNII: T5L8T28FGP) Paraffin (UNII: I9O0E3H2ZE) Phenoxyethanol (UNII: HIE492ZZ3T) PPG-15 Stearyl Ether (UNII: 1II18XLS1L) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17518-030-06 0.00126 L in 1 TUBE; Type 0: Not a Combination Product 01/01/2017 2 NDC:17518-030-05 0.000383 L in 1 TUBE; Type 0: Not a Combination Product 01/01/2017 3 NDC:17518-030-04 0.0000273 L in 1 PACKET; Type 0: Not a Combination Product 01/01/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 01/01/2017 Labeler - Solventum US OpCo LLC (006173082)