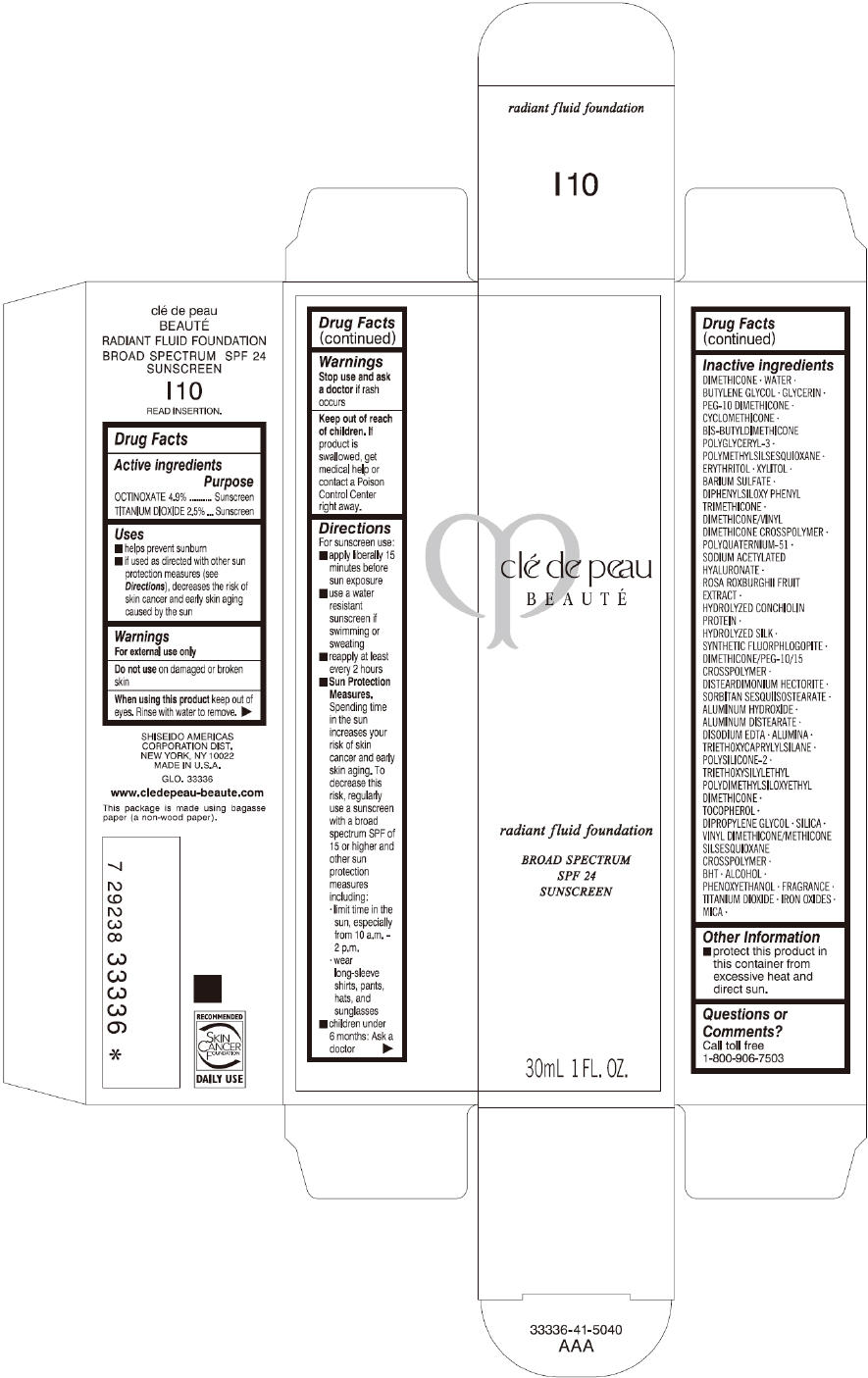
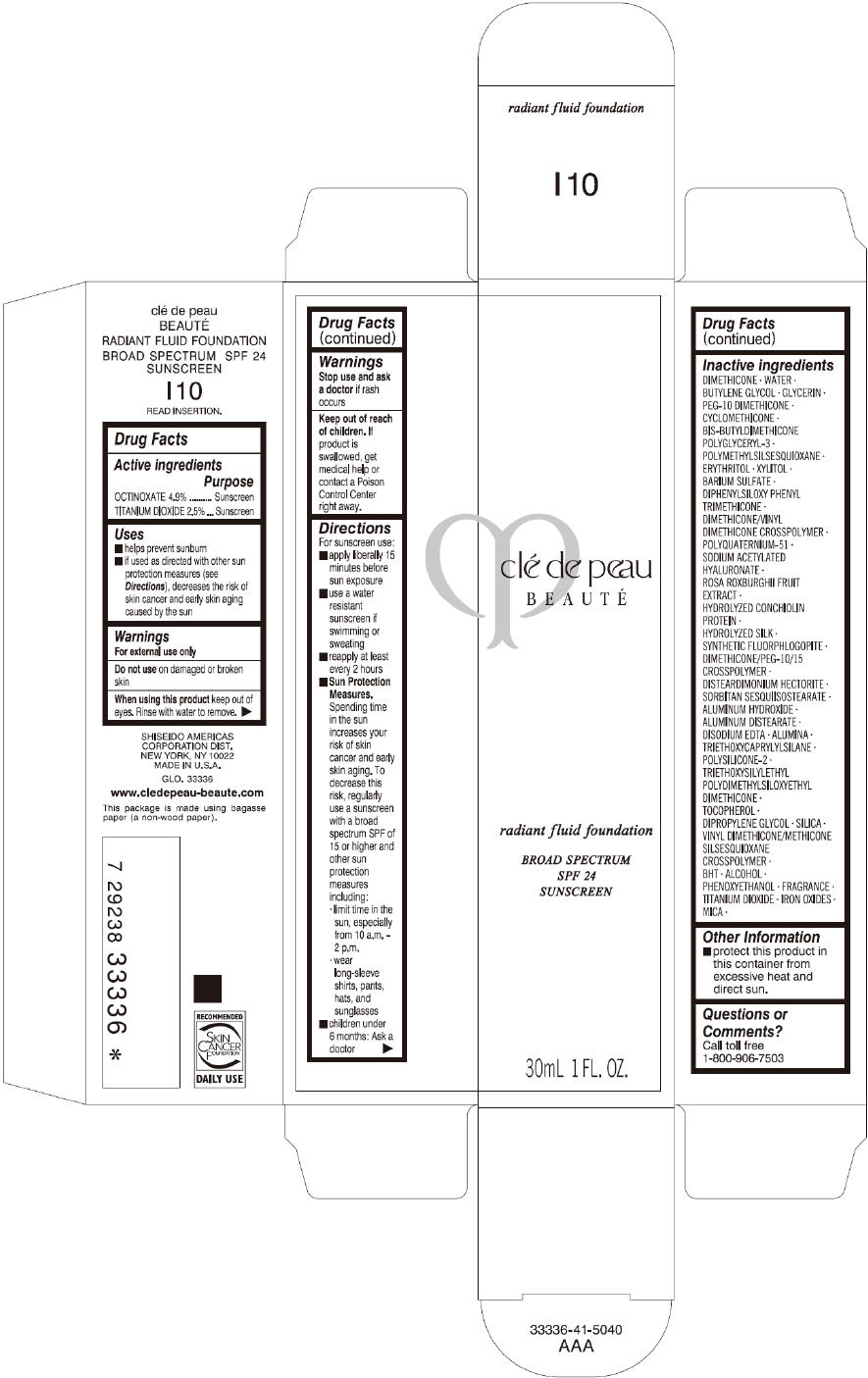
Label: CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION I10- octinoxate and titanium dioxide cream
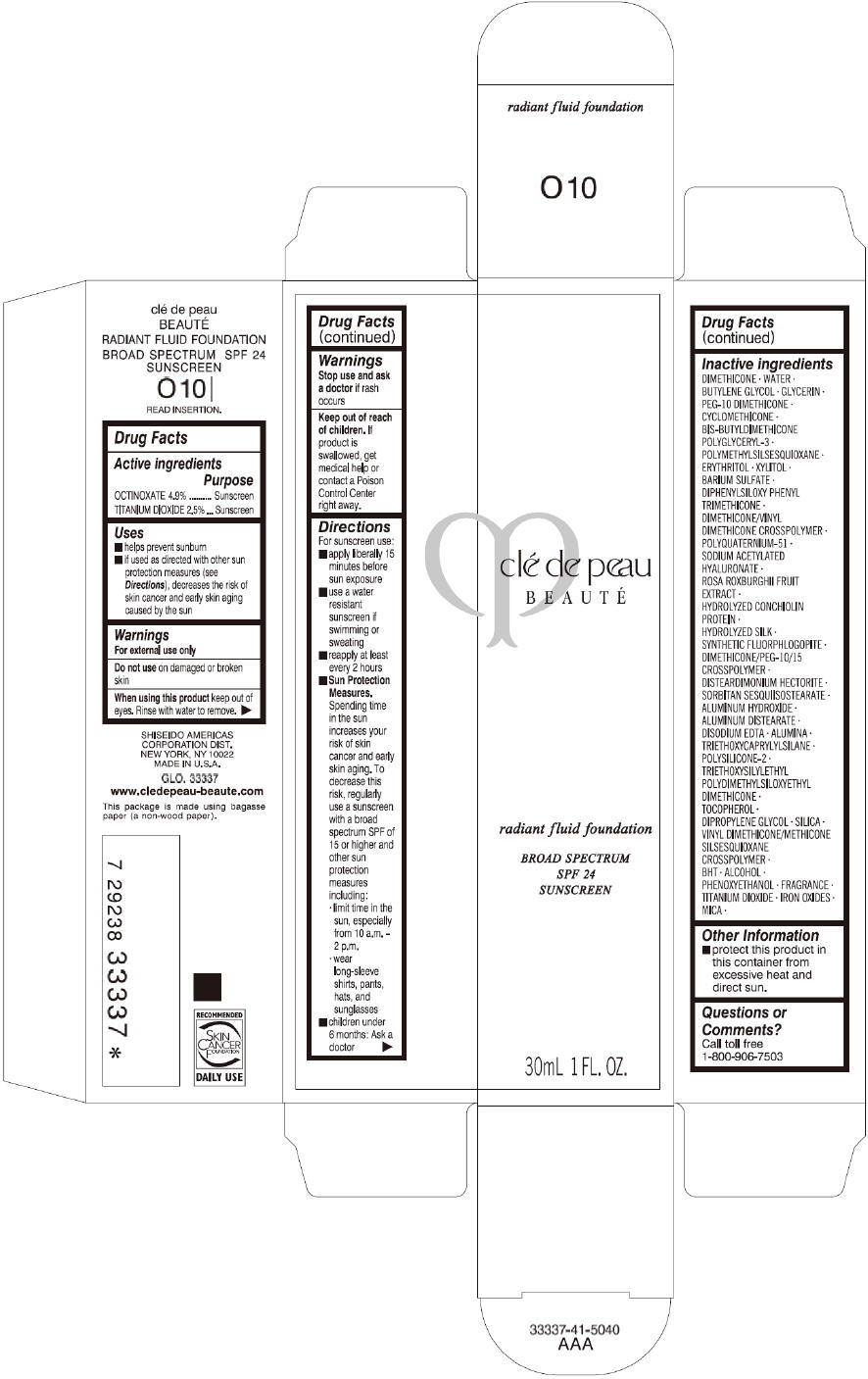
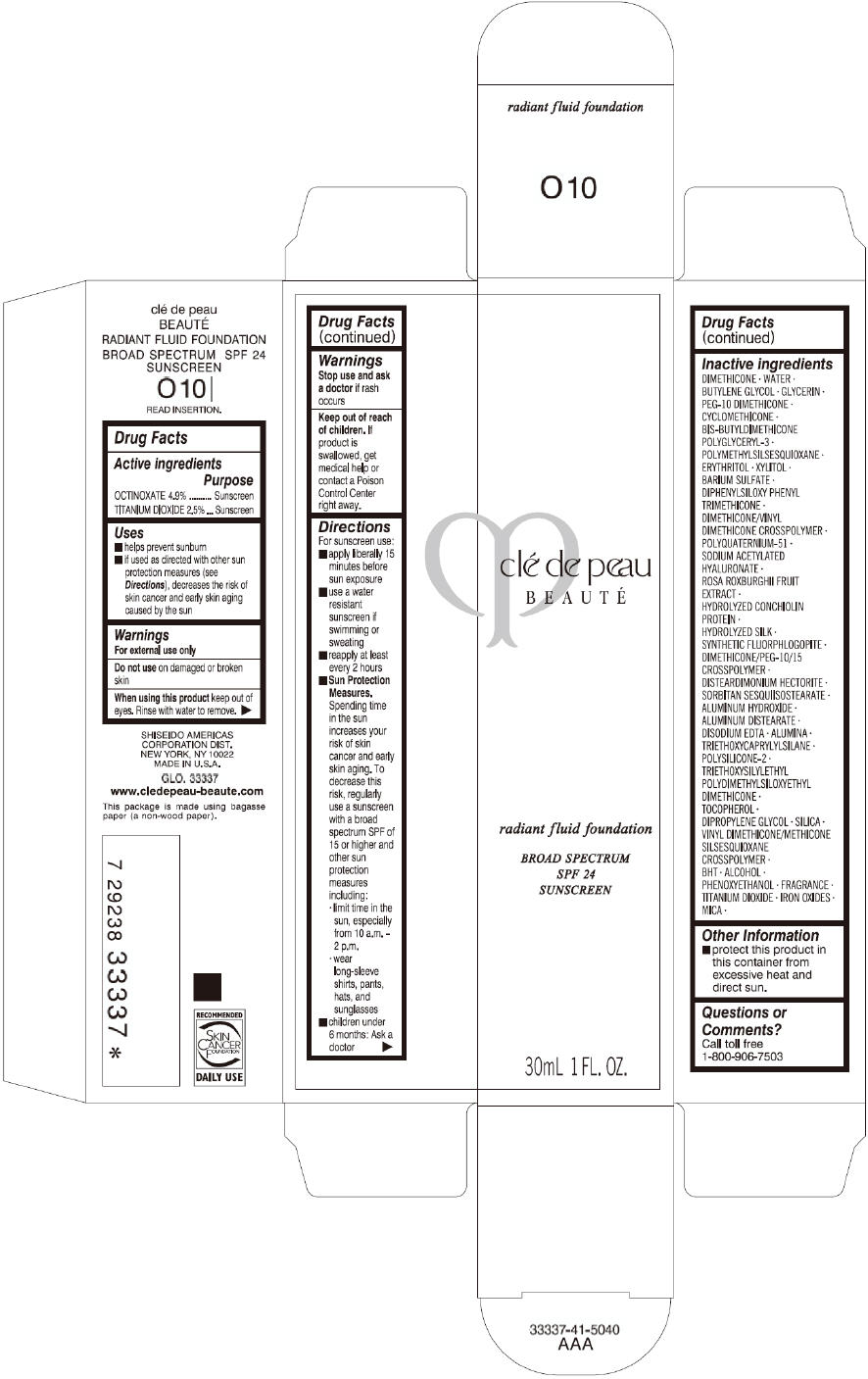
CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION O10- octinoxate and titanium dioxide cream
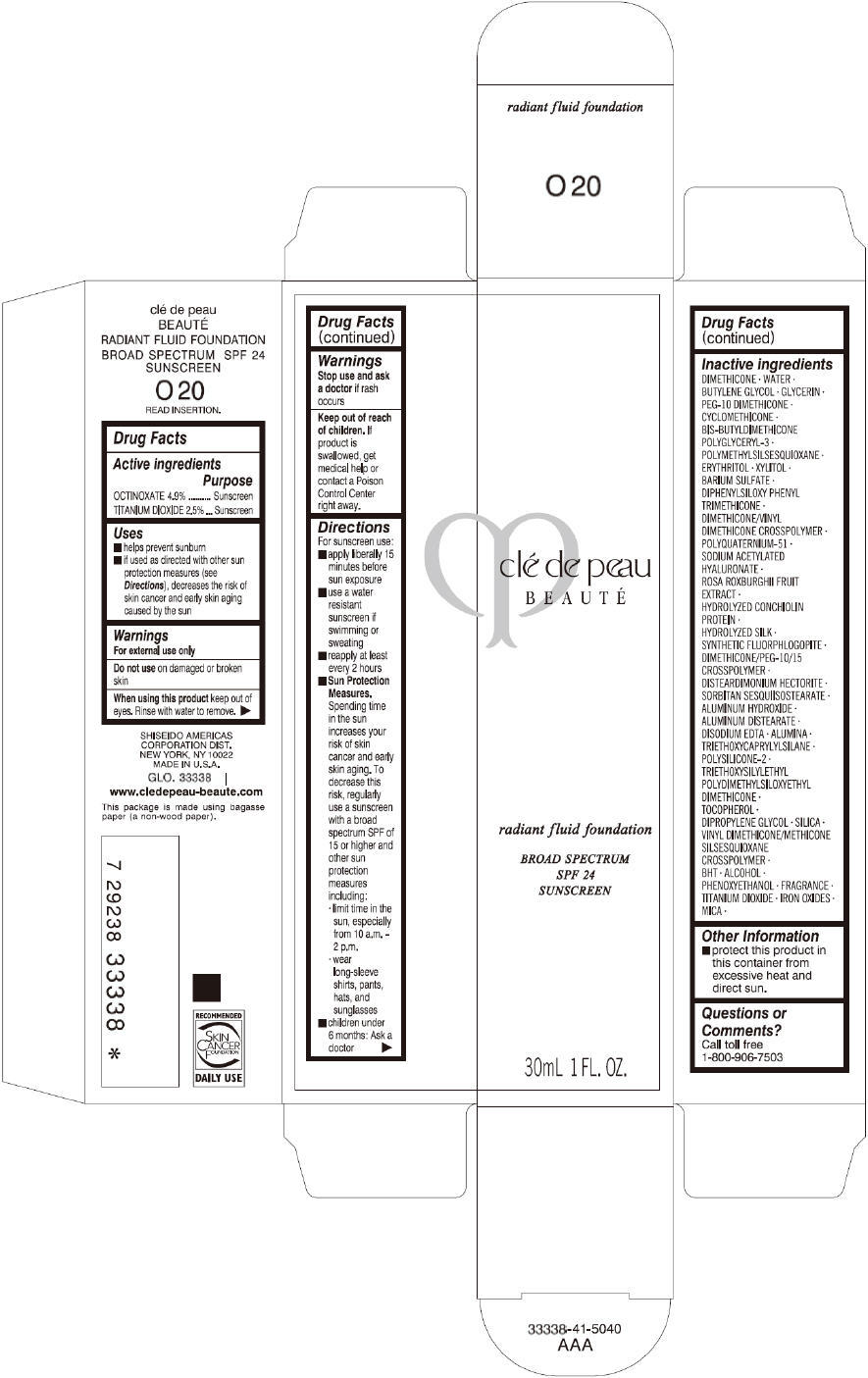
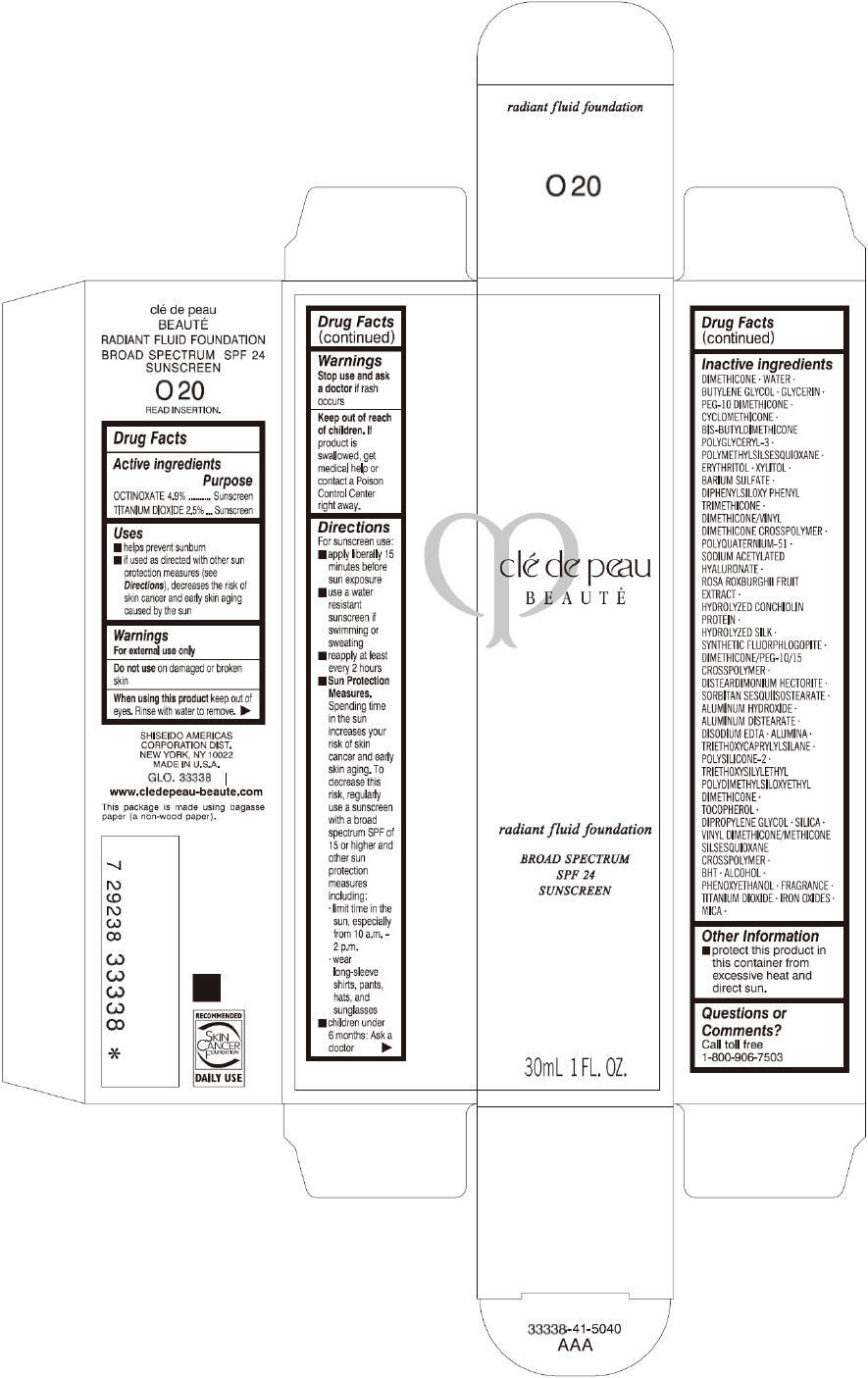
CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION O20- octinoxate and titanium dioxide cream
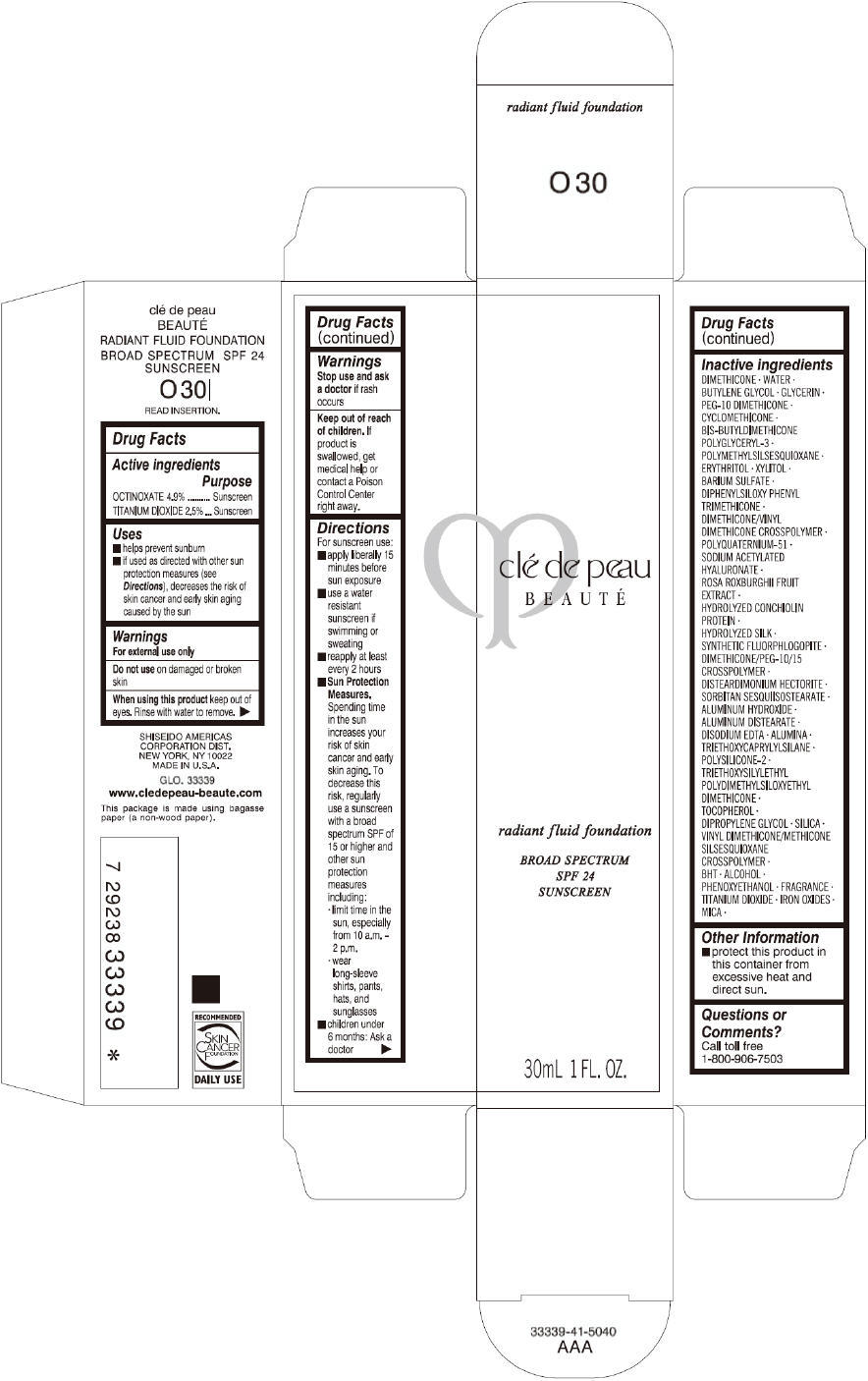
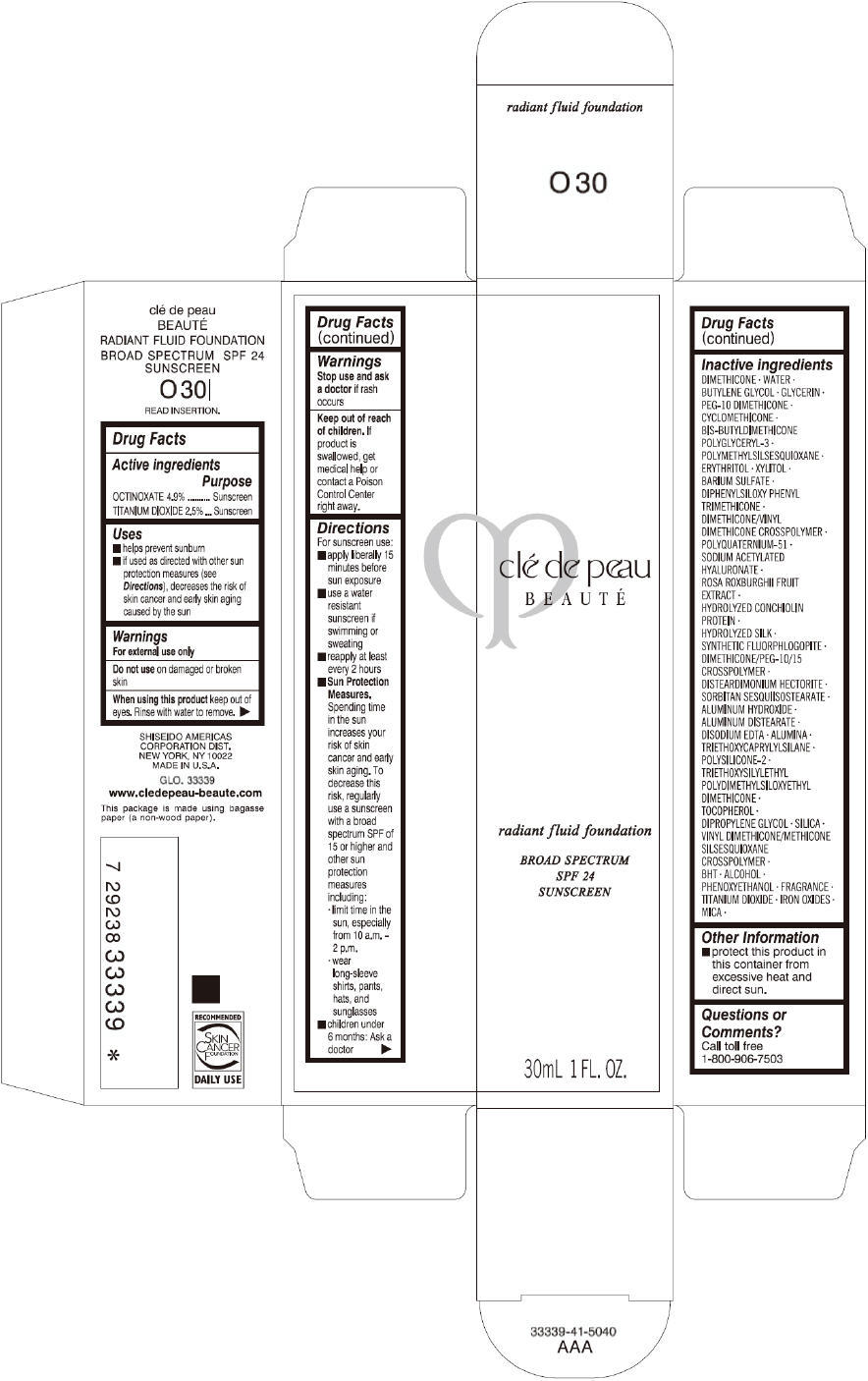
CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION O30- octinoxate and titanium dioxide cream
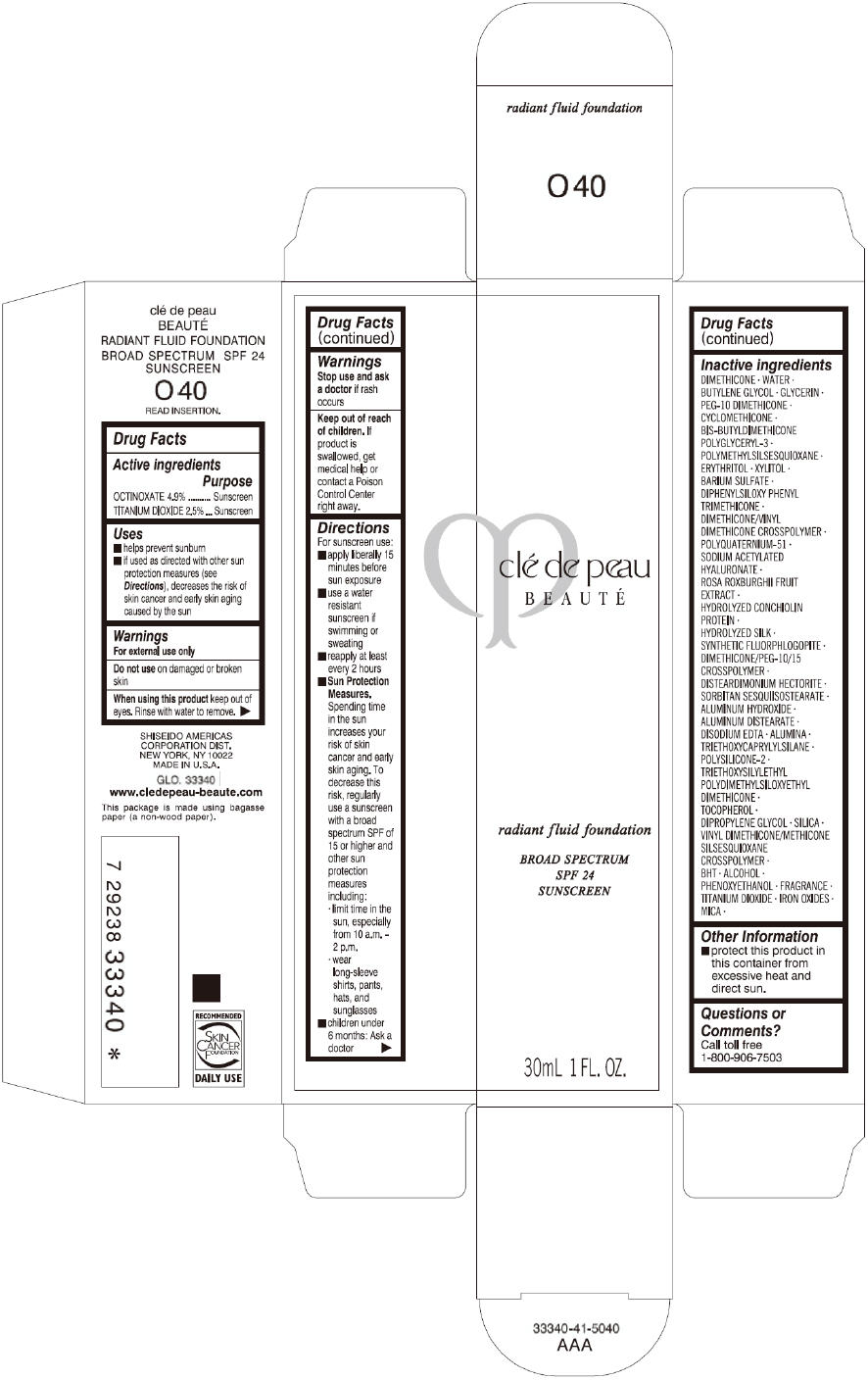
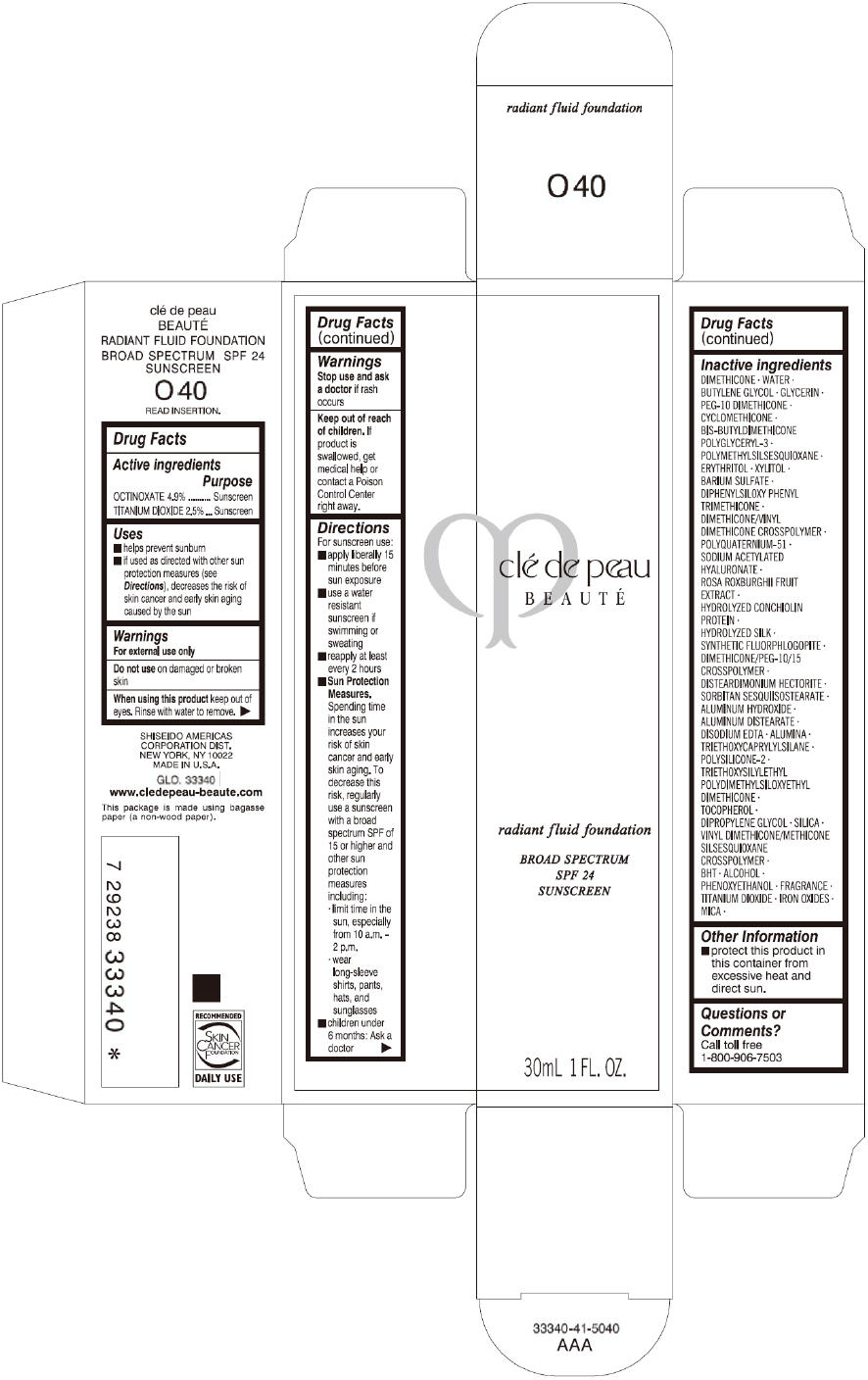
CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION O40- octinoxate and titanium dioxide cream
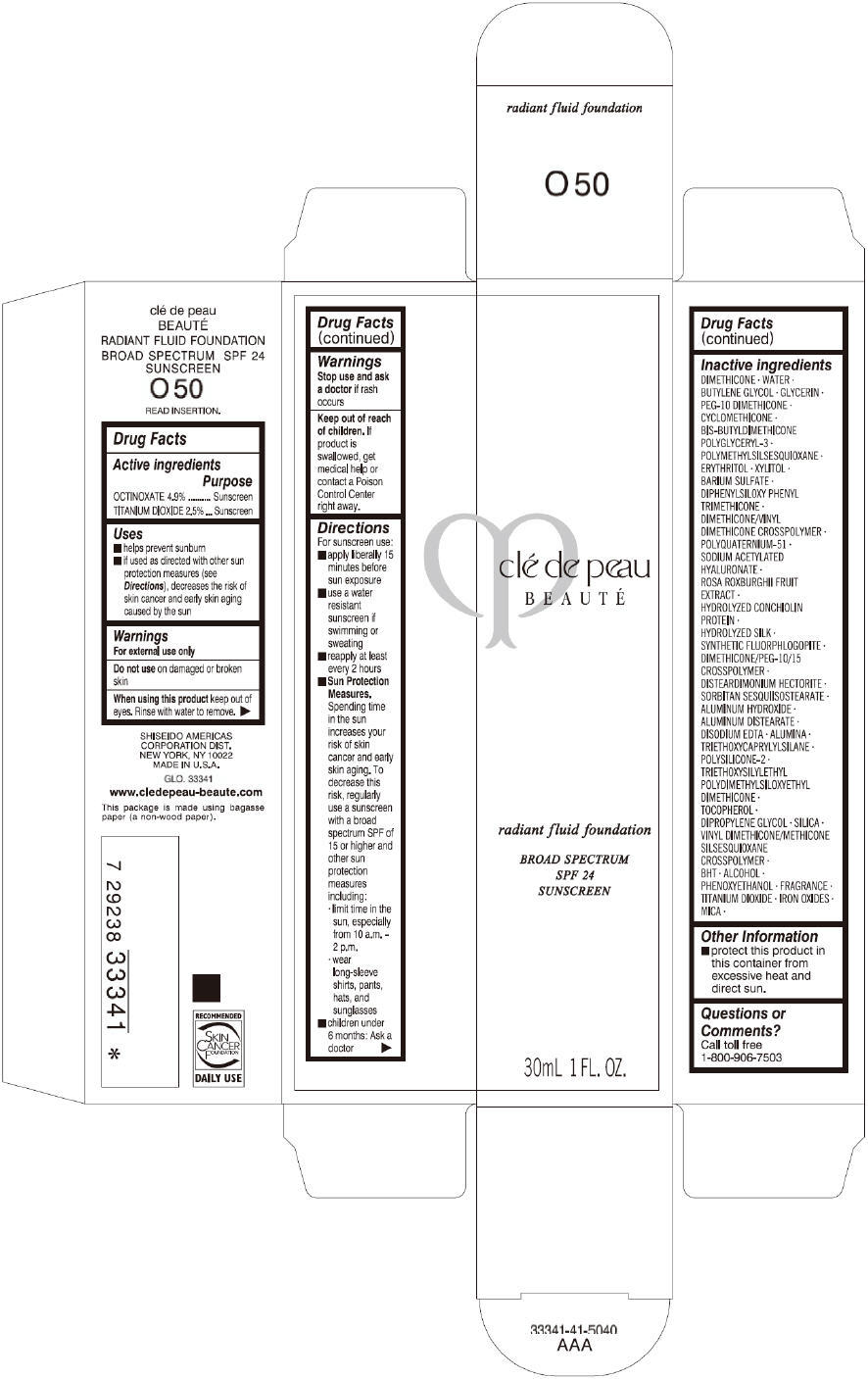
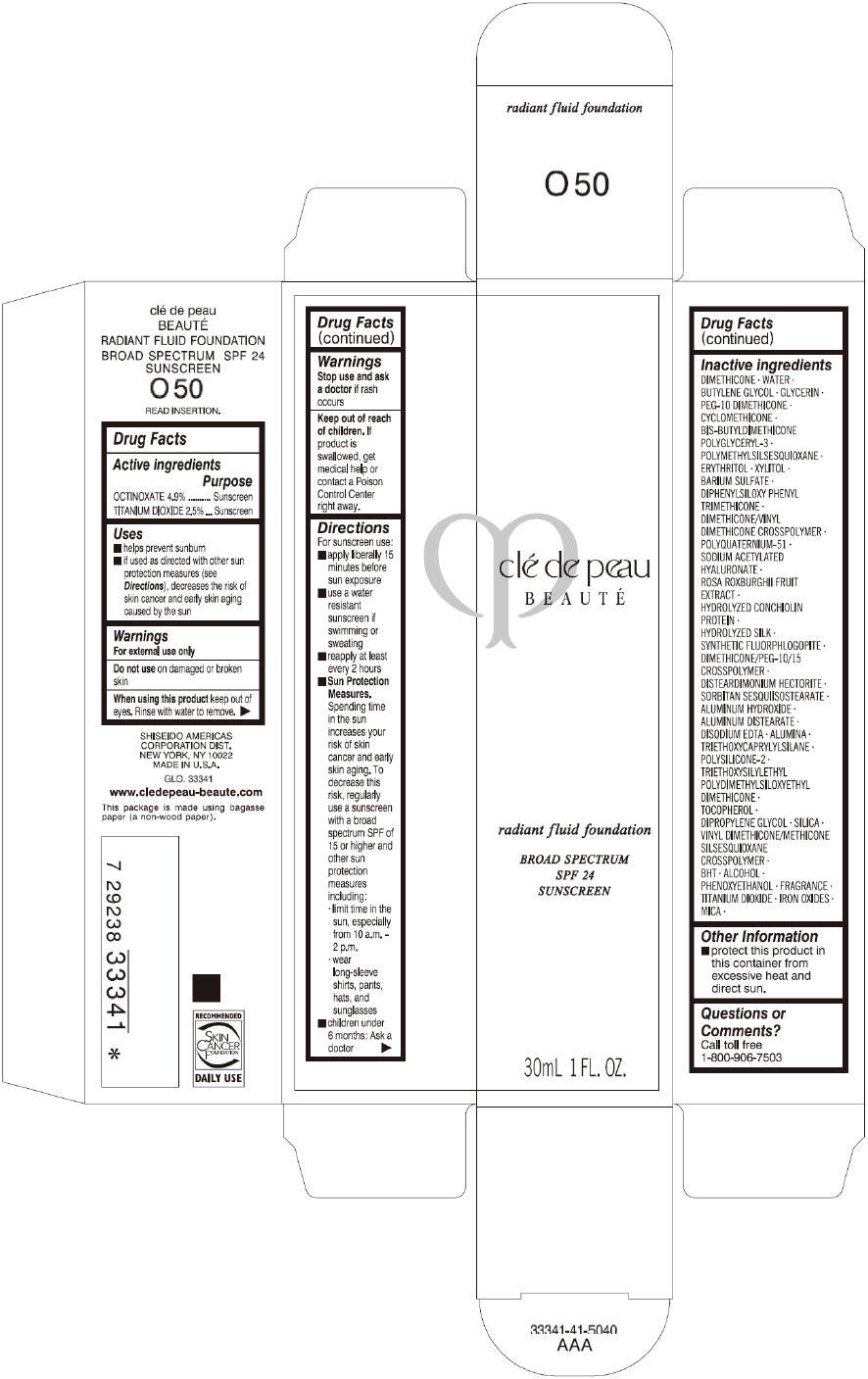
CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION O50- octinoxate and titanium dioxide cream
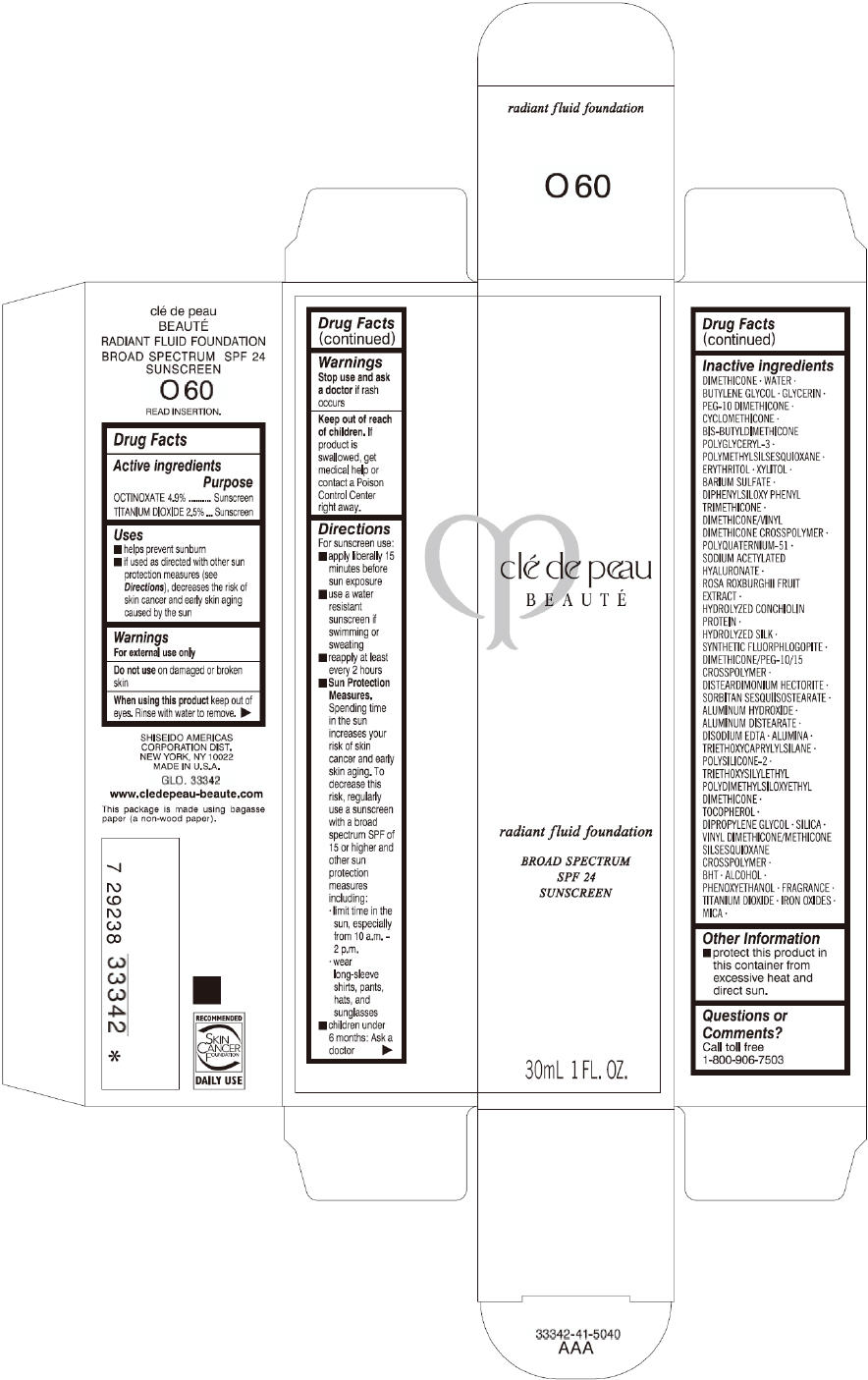
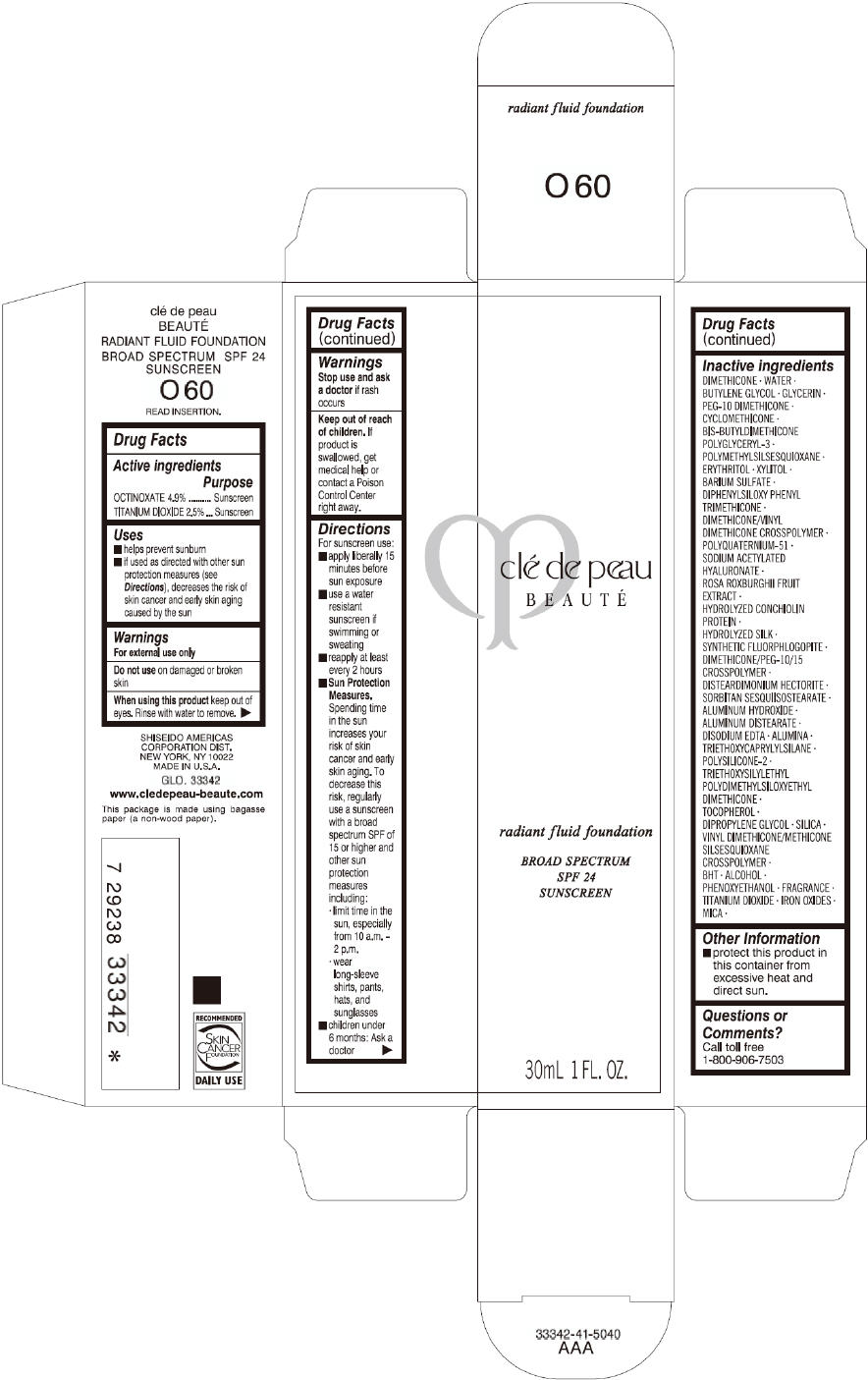
CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION O60- octinoxate and titanium dioxide cream
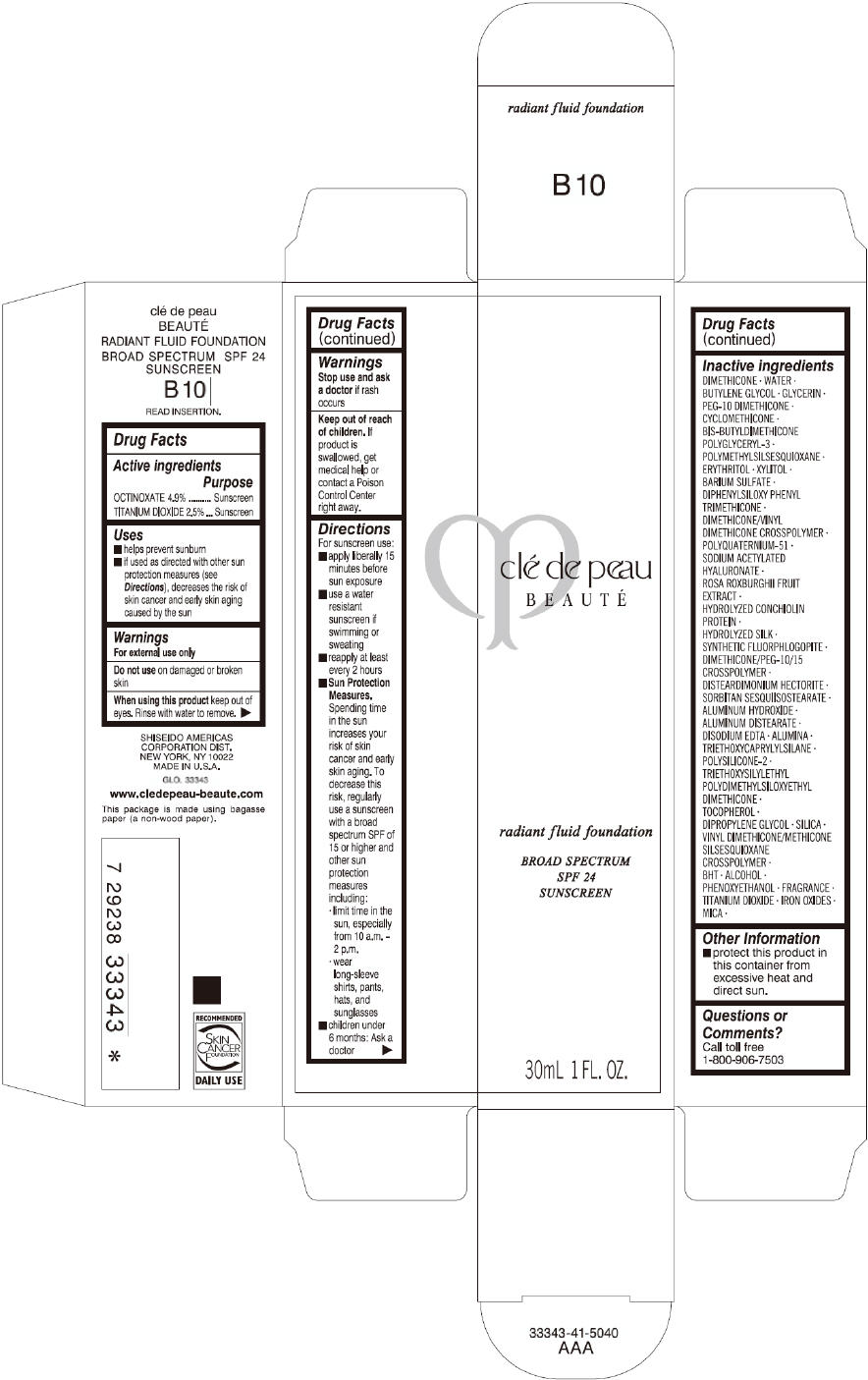
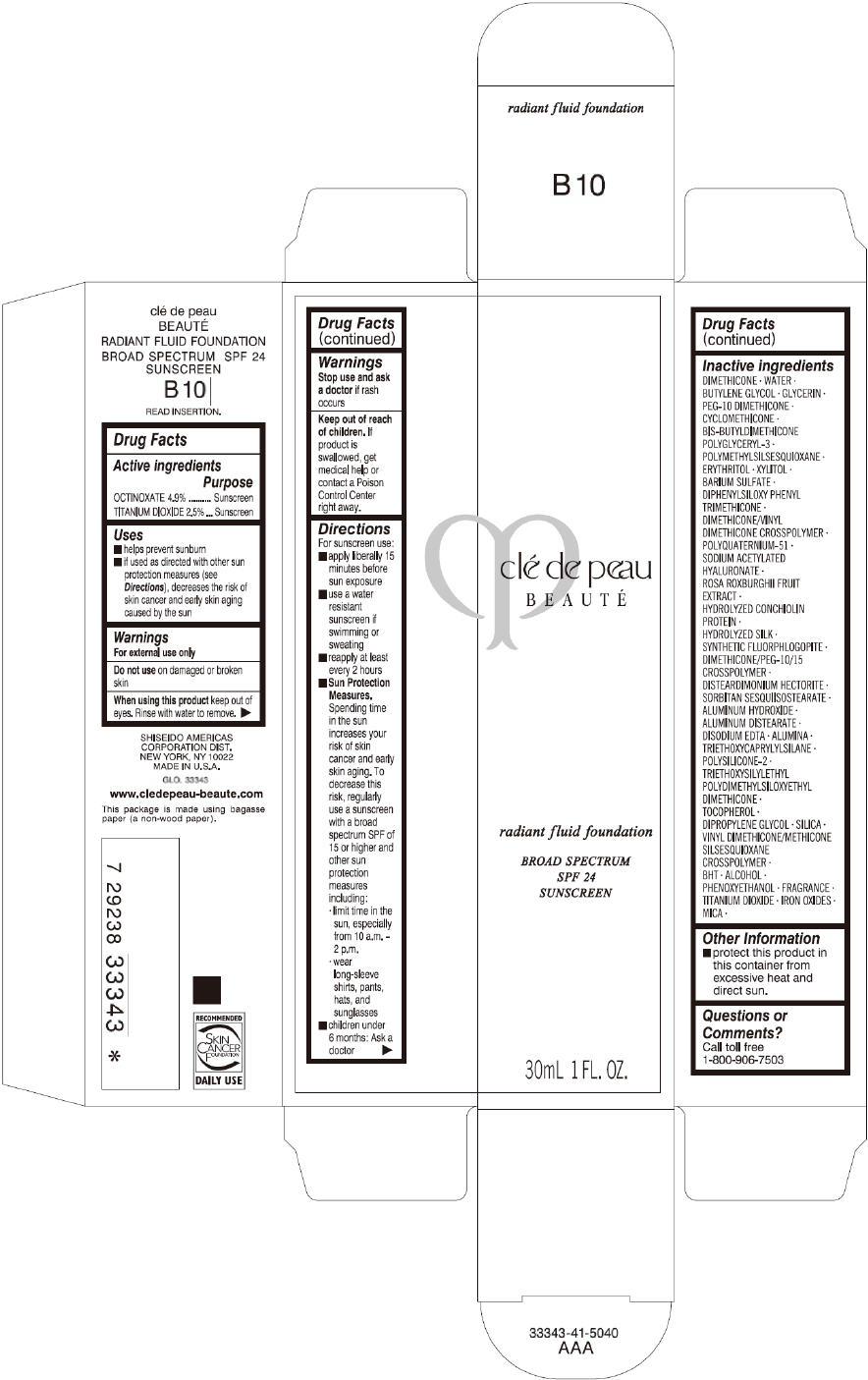
CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION B10- octinoxate and titanium dioxide cream
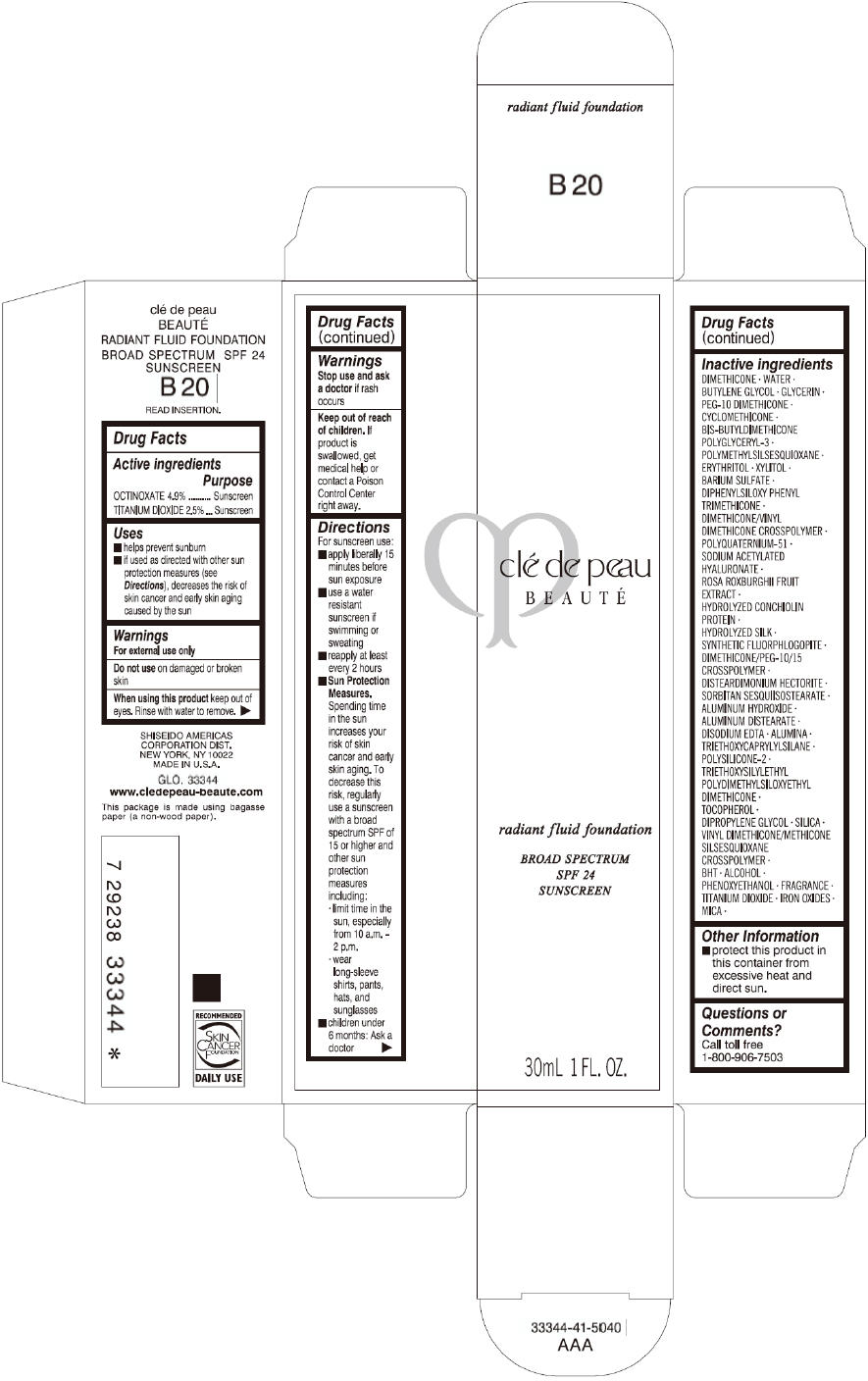
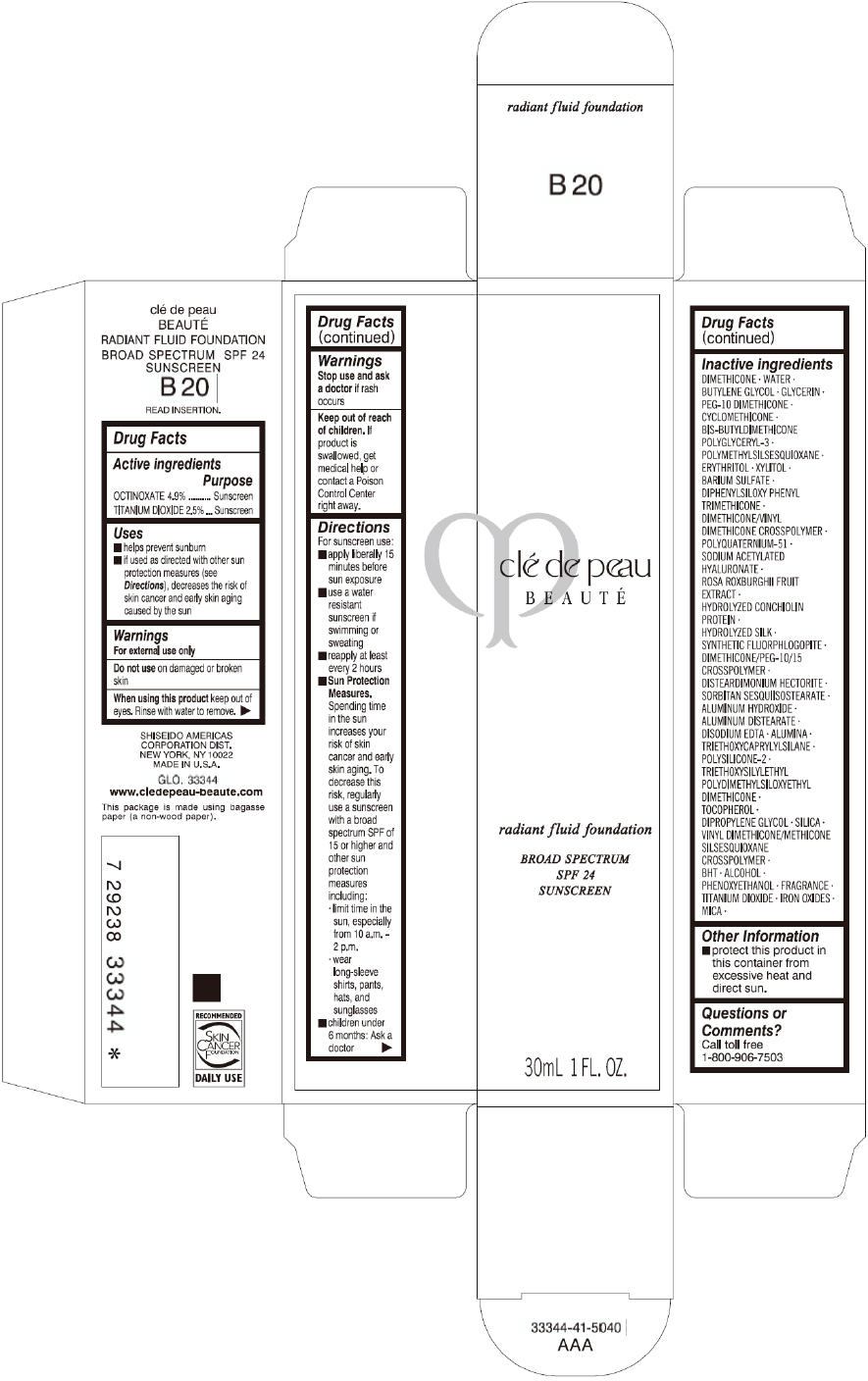
CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION B20- octinoxate and titanium dioxide cream
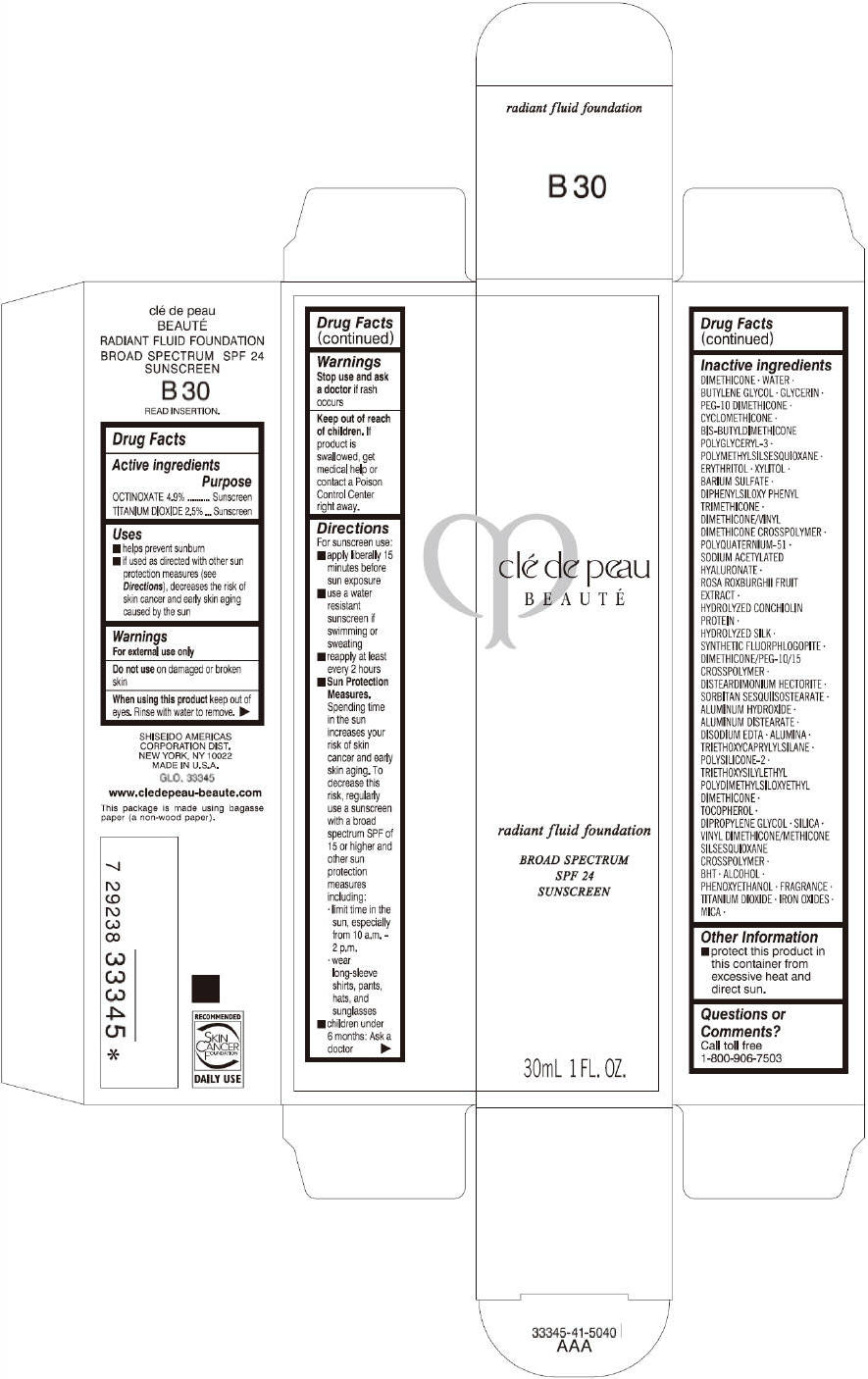
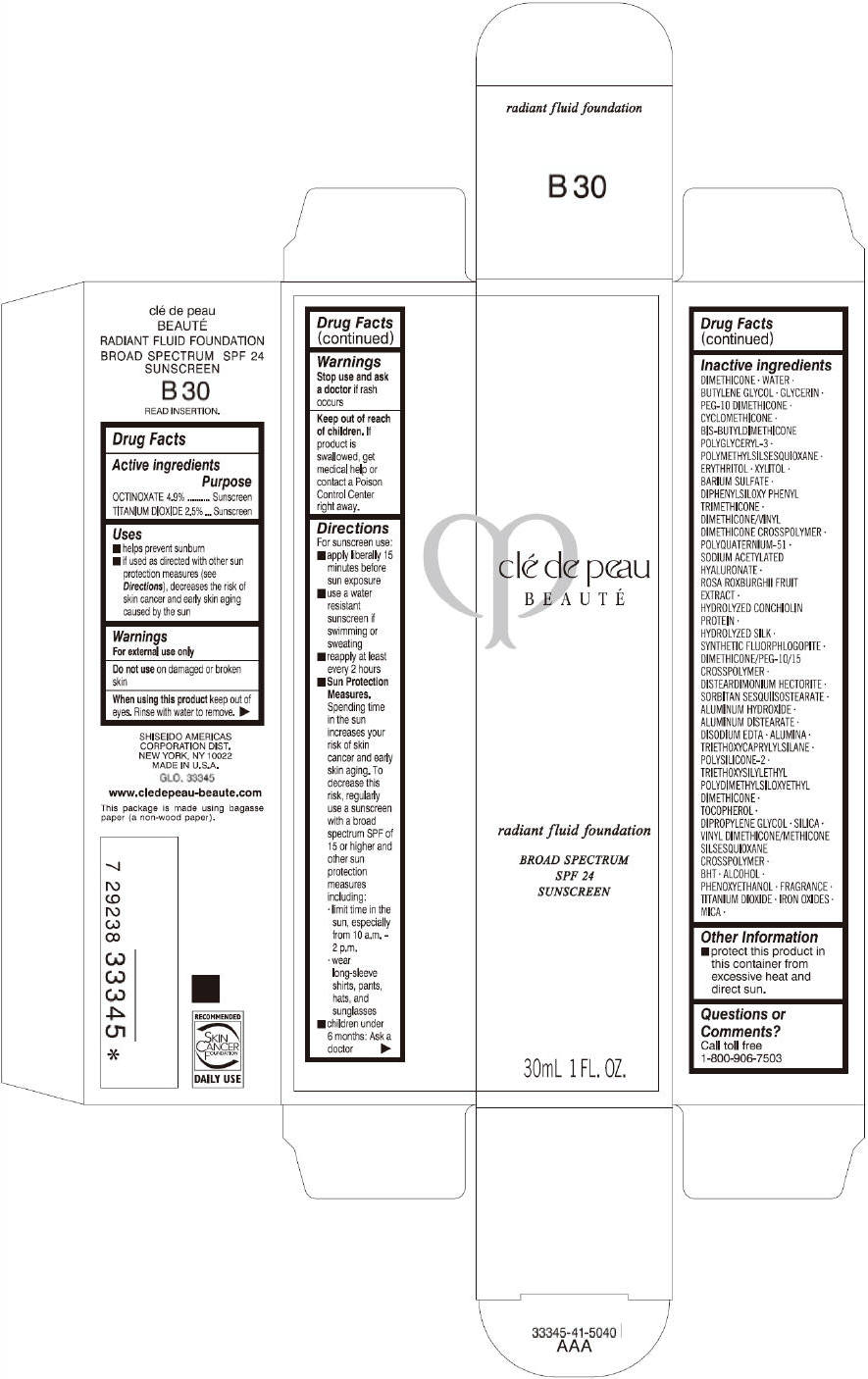
CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION B30- octinoxate and titanium dioxide cream
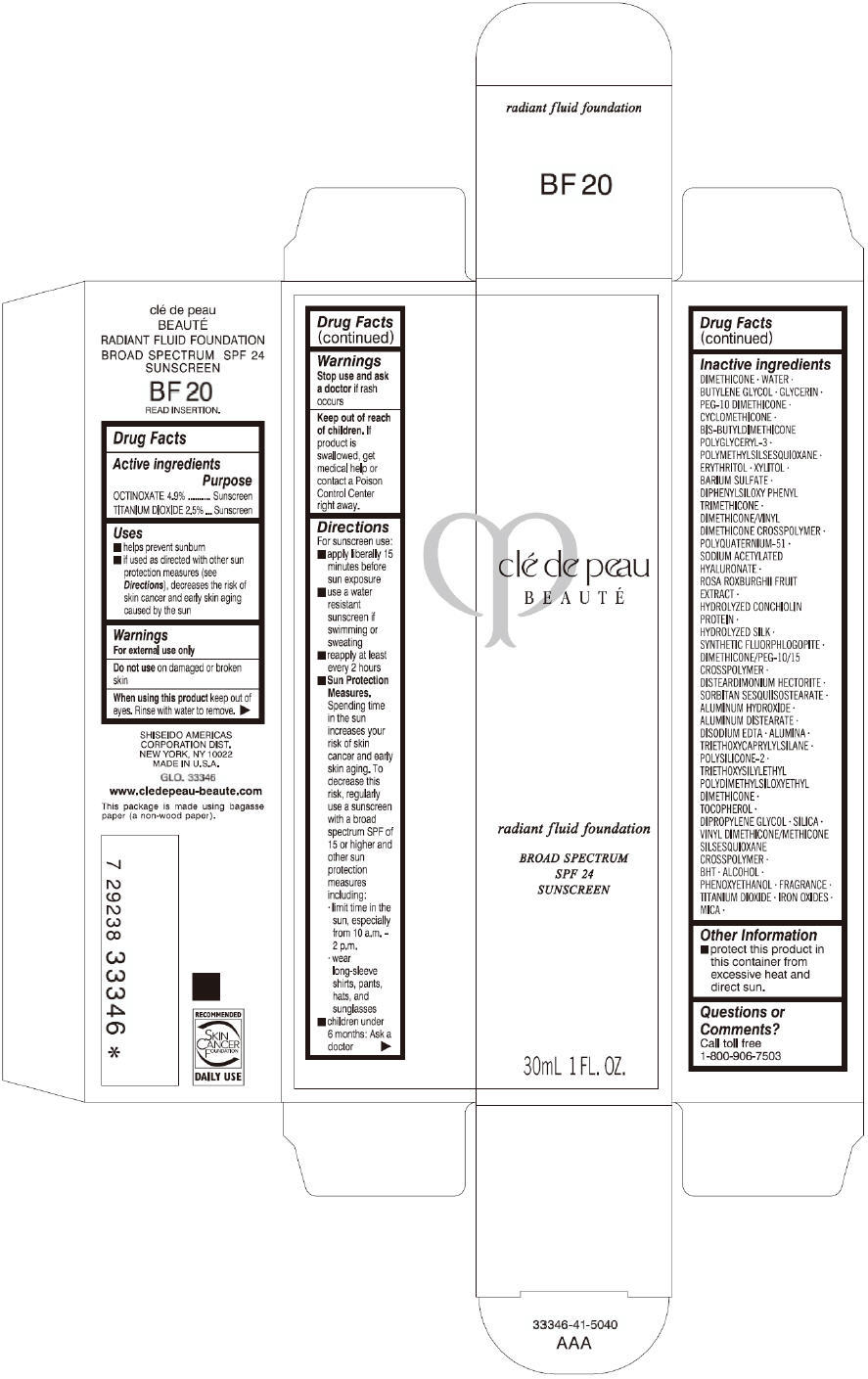
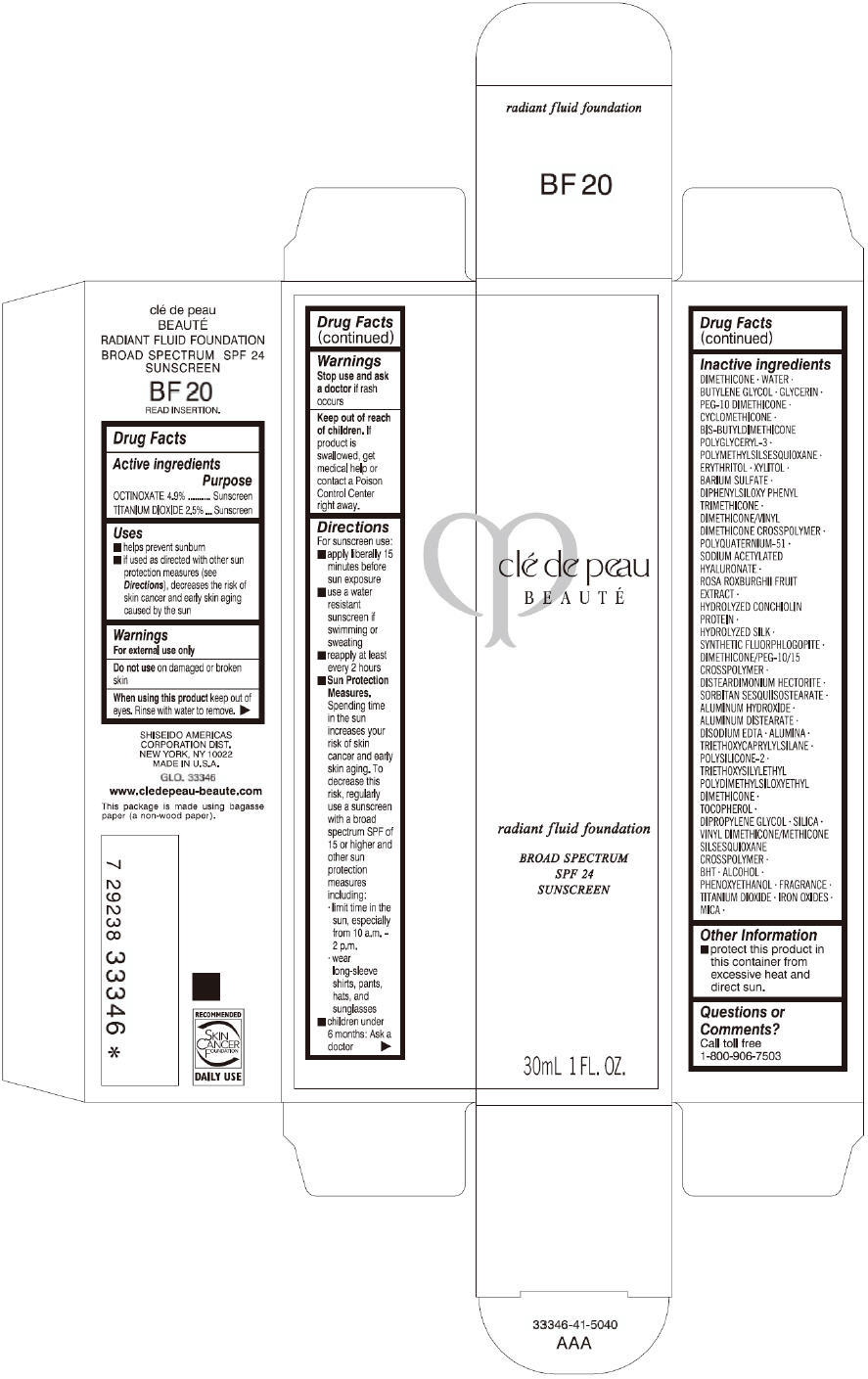
CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION BF20- octinoxate and titanium dioxide cream
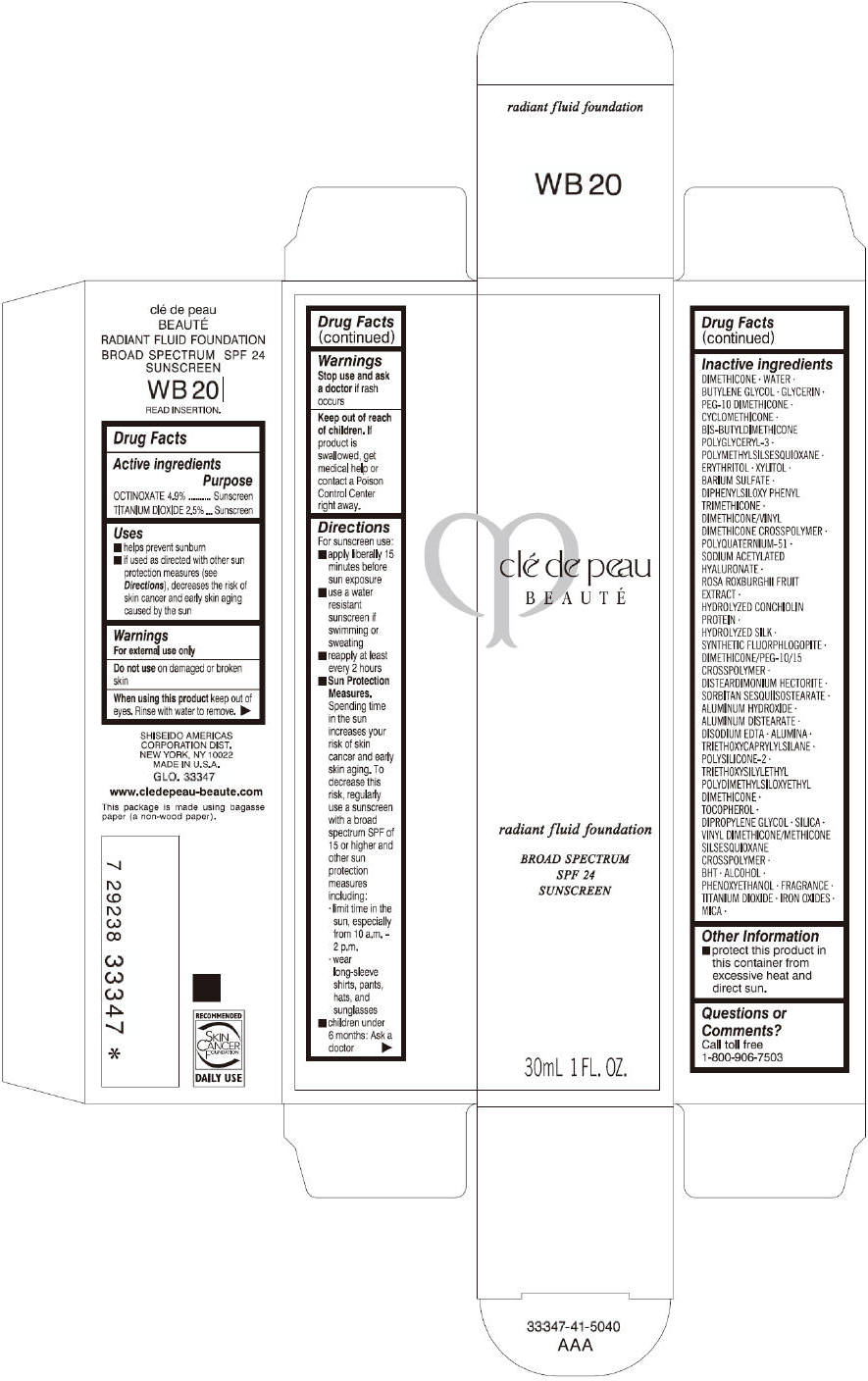
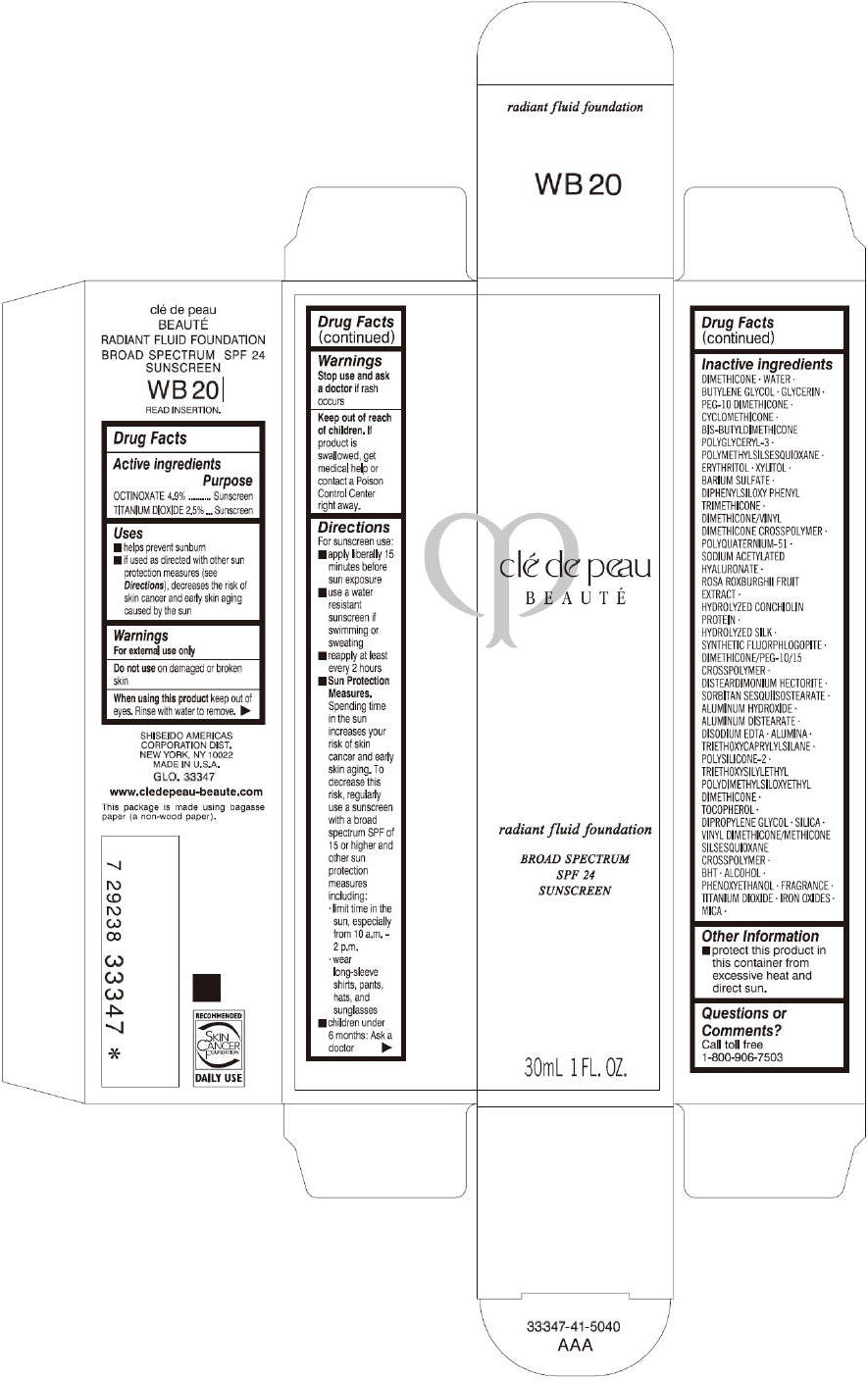
CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION WB20- octinoxate and titanium dioxide cream
-
NDC Code(s):
58411-193-60,
58411-194-60,
58411-195-60,
58411-196-60, view more58411-197-60, 58411-198-60, 58411-199-60, 58411-200-60, 58411-201-60, 58411-202-60, 58411-203-60, 58411-204-60
- Packager: SHISEIDO AMERICAS CORPORATION
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
-
Inactive Ingredients
DIMETHICONE∙WATER∙BUTYLENE GLYCOL∙GLYCERIN∙PEG-10 DIMETHICONE∙CYCLOMETHICONE∙BIS-BUTYLDIMETHICONE POLYGLYCERYL-3∙POLYMETHYLSILSESQUIOXANE∙ERYTHRITOL∙XYLITOL∙BARIUM SULFATE∙DIPHENYLSILOXY PHENYL TRIMETHICONE∙DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER∙POLYQUATERNIUM-51∙SODIUM ACETYLATED HYALURONATE∙ROSA ROXBURGHII FRUIT EXTRACT∙HYDROLYZED CONCHIOLIN PROTEIN∙HYDROLYZED SILK∙SYNTHETIC FLUORPHLOGOPITE∙DIMETHICONE/PEG-10/15 CROSSPOLYMER∙DISTEARDIMONIUM HECTORITE∙SORBITAN SESQUIISOSTEARATE∙ALUMINUM HYDROXIDE∙ALUMINUM DISTEARATE∙DISODIUM EDTA∙ALUMINA∙TRIETHOXYCAPRYLYLSILANE∙POLYSILICONE-2∙TRIETHOXYSILYLETHYL POLYDIMETHYLSILOXYETHYL DIMETHICONE∙TOCOPHEROL∙DIPROPYLENE GLYCOL∙SILICA∙VINYL DIMETHICONE/METHICONE SILSESQUIOXANE CROSSPOLYMER∙BHT∙ALCOHOL∙PHENOXYETHANOL∙FRAGRANCE∙TITANIUM DIOXIDE∙IRON OXIDES∙MICA∙
- Other information
- Questions or comments?
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - I10
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - O10
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - O20
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - O30
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - O40
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - O50
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - O60
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - B10
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - B20
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - B30
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - BF20
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - WB20
-
INGREDIENTS AND APPEARANCE
CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION I10
octinoxate and titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-193 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1617 mg in 30 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 825 mg in 30 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) CYCLOMETHICONE (UNII: NMQ347994Z) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) ERYTHRITOL (UNII: RA96B954X6) XYLITOL (UNII: VCQ006KQ1E) BARIUM SULFATE (UNII: 25BB7EKE2E) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) ROSA ROXBURGHII FRUIT (UNII: CVT1AA87FF) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) ALUMINUM DISTEARATE (UNII: 7P1HP1B9UI) EDETATE DISODIUM (UNII: 7FLD91C86K) ALUMINUM OXIDE (UNII: LMI26O6933) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) DIPROPYLENE GLYCOL (UNII: E107L85C40) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ALCOHOL (UNII: 3K9958V90M) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICA (UNII: V8A1AW0880) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-193-60 1 in 1 CARTON 09/01/2014 09/01/2023 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 09/01/2014 09/01/2023 CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION O10
octinoxate and titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-194 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1617 mg in 30 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 825 mg in 30 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) CYCLOMETHICONE (UNII: NMQ347994Z) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) ERYTHRITOL (UNII: RA96B954X6) XYLITOL (UNII: VCQ006KQ1E) BARIUM SULFATE (UNII: 25BB7EKE2E) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) ROSA ROXBURGHII FRUIT (UNII: CVT1AA87FF) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) ALUMINUM DISTEARATE (UNII: 7P1HP1B9UI) EDETATE DISODIUM (UNII: 7FLD91C86K) ALUMINUM OXIDE (UNII: LMI26O6933) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) DIPROPYLENE GLYCOL (UNII: E107L85C40) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ALCOHOL (UNII: 3K9958V90M) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICA (UNII: V8A1AW0880) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-194-60 1 in 1 CARTON 09/01/2014 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 09/01/2014 CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION O20
octinoxate and titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-195 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1617 mg in 30 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 825 mg in 30 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) CYCLOMETHICONE (UNII: NMQ347994Z) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) ERYTHRITOL (UNII: RA96B954X6) XYLITOL (UNII: VCQ006KQ1E) BARIUM SULFATE (UNII: 25BB7EKE2E) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) ROSA ROXBURGHII FRUIT (UNII: CVT1AA87FF) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) ALUMINUM DISTEARATE (UNII: 7P1HP1B9UI) EDETATE DISODIUM (UNII: 7FLD91C86K) ALUMINUM OXIDE (UNII: LMI26O6933) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) DIPROPYLENE GLYCOL (UNII: E107L85C40) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ALCOHOL (UNII: 3K9958V90M) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICA (UNII: V8A1AW0880) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-195-60 1 in 1 CARTON 09/01/2014 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 09/01/2014 CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION O30
octinoxate and titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-196 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1617 mg in 30 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 825 mg in 30 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) CYCLOMETHICONE (UNII: NMQ347994Z) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) ERYTHRITOL (UNII: RA96B954X6) XYLITOL (UNII: VCQ006KQ1E) BARIUM SULFATE (UNII: 25BB7EKE2E) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) ROSA ROXBURGHII FRUIT (UNII: CVT1AA87FF) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) ALUMINUM DISTEARATE (UNII: 7P1HP1B9UI) EDETATE DISODIUM (UNII: 7FLD91C86K) ALUMINUM OXIDE (UNII: LMI26O6933) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) DIPROPYLENE GLYCOL (UNII: E107L85C40) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ALCOHOL (UNII: 3K9958V90M) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICA (UNII: V8A1AW0880) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-196-60 1 in 1 CARTON 09/01/2014 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 09/01/2014 CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION O40
octinoxate and titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-197 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1617 mg in 30 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 825 mg in 30 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) CYCLOMETHICONE (UNII: NMQ347994Z) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) ERYTHRITOL (UNII: RA96B954X6) XYLITOL (UNII: VCQ006KQ1E) BARIUM SULFATE (UNII: 25BB7EKE2E) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) ROSA ROXBURGHII FRUIT (UNII: CVT1AA87FF) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) ALUMINUM DISTEARATE (UNII: 7P1HP1B9UI) EDETATE DISODIUM (UNII: 7FLD91C86K) ALUMINUM OXIDE (UNII: LMI26O6933) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) DIPROPYLENE GLYCOL (UNII: E107L85C40) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ALCOHOL (UNII: 3K9958V90M) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICA (UNII: V8A1AW0880) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-197-60 1 in 1 CARTON 09/01/2014 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 09/01/2014 CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION O50
octinoxate and titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-198 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1617 mg in 30 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 825 mg in 30 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) CYCLOMETHICONE (UNII: NMQ347994Z) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) ERYTHRITOL (UNII: RA96B954X6) XYLITOL (UNII: VCQ006KQ1E) BARIUM SULFATE (UNII: 25BB7EKE2E) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) ROSA ROXBURGHII FRUIT (UNII: CVT1AA87FF) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) ALUMINUM DISTEARATE (UNII: 7P1HP1B9UI) EDETATE DISODIUM (UNII: 7FLD91C86K) ALUMINUM OXIDE (UNII: LMI26O6933) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) DIPROPYLENE GLYCOL (UNII: E107L85C40) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ALCOHOL (UNII: 3K9958V90M) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICA (UNII: V8A1AW0880) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-198-60 1 in 1 CARTON 09/01/2014 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 09/01/2014 CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION O60
octinoxate and titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-199 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1617 mg in 30 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 825 mg in 30 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) CYCLOMETHICONE (UNII: NMQ347994Z) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) ERYTHRITOL (UNII: RA96B954X6) XYLITOL (UNII: VCQ006KQ1E) BARIUM SULFATE (UNII: 25BB7EKE2E) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) ROSA ROXBURGHII FRUIT (UNII: CVT1AA87FF) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) ALUMINUM DISTEARATE (UNII: 7P1HP1B9UI) EDETATE DISODIUM (UNII: 7FLD91C86K) ALUMINUM OXIDE (UNII: LMI26O6933) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) DIPROPYLENE GLYCOL (UNII: E107L85C40) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ALCOHOL (UNII: 3K9958V90M) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICA (UNII: V8A1AW0880) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-199-60 1 in 1 CARTON 09/01/2014 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 09/01/2014 CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION B10
octinoxate and titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-200 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1617 mg in 30 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 825 mg in 30 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) CYCLOMETHICONE (UNII: NMQ347994Z) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) ERYTHRITOL (UNII: RA96B954X6) XYLITOL (UNII: VCQ006KQ1E) BARIUM SULFATE (UNII: 25BB7EKE2E) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) ROSA ROXBURGHII FRUIT (UNII: CVT1AA87FF) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) ALUMINUM DISTEARATE (UNII: 7P1HP1B9UI) EDETATE DISODIUM (UNII: 7FLD91C86K) ALUMINUM OXIDE (UNII: LMI26O6933) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) DIPROPYLENE GLYCOL (UNII: E107L85C40) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ALCOHOL (UNII: 3K9958V90M) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICA (UNII: V8A1AW0880) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-200-60 1 in 1 CARTON 09/01/2014 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 09/01/2014 CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION B20
octinoxate and titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-201 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1617 mg in 30 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 825 mg in 30 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) CYCLOMETHICONE (UNII: NMQ347994Z) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) ERYTHRITOL (UNII: RA96B954X6) XYLITOL (UNII: VCQ006KQ1E) BARIUM SULFATE (UNII: 25BB7EKE2E) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) ROSA ROXBURGHII FRUIT (UNII: CVT1AA87FF) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) ALUMINUM DISTEARATE (UNII: 7P1HP1B9UI) EDETATE DISODIUM (UNII: 7FLD91C86K) ALUMINUM OXIDE (UNII: LMI26O6933) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) DIPROPYLENE GLYCOL (UNII: E107L85C40) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ALCOHOL (UNII: 3K9958V90M) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICA (UNII: V8A1AW0880) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-201-60 1 in 1 CARTON 09/01/2014 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 09/01/2014 CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION B30
octinoxate and titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-202 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1617 mg in 30 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 825 mg in 30 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) CYCLOMETHICONE (UNII: NMQ347994Z) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) ERYTHRITOL (UNII: RA96B954X6) XYLITOL (UNII: VCQ006KQ1E) BARIUM SULFATE (UNII: 25BB7EKE2E) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) ROSA ROXBURGHII FRUIT (UNII: CVT1AA87FF) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) ALUMINUM DISTEARATE (UNII: 7P1HP1B9UI) EDETATE DISODIUM (UNII: 7FLD91C86K) ALUMINUM OXIDE (UNII: LMI26O6933) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) DIPROPYLENE GLYCOL (UNII: E107L85C40) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ALCOHOL (UNII: 3K9958V90M) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICA (UNII: V8A1AW0880) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-202-60 1 in 1 CARTON 09/01/2014 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 09/01/2014 CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION BF20
octinoxate and titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-203 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1617 mg in 30 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 825 mg in 30 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) CYCLOMETHICONE (UNII: NMQ347994Z) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) ERYTHRITOL (UNII: RA96B954X6) XYLITOL (UNII: VCQ006KQ1E) BARIUM SULFATE (UNII: 25BB7EKE2E) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) ROSA ROXBURGHII FRUIT (UNII: CVT1AA87FF) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) ALUMINUM DISTEARATE (UNII: 7P1HP1B9UI) EDETATE DISODIUM (UNII: 7FLD91C86K) ALUMINUM OXIDE (UNII: LMI26O6933) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) DIPROPYLENE GLYCOL (UNII: E107L85C40) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ALCOHOL (UNII: 3K9958V90M) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICA (UNII: V8A1AW0880) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-203-60 1 in 1 CARTON 09/01/2014 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 09/01/2014 CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION WB20
octinoxate and titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-204 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1617 mg in 30 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 825 mg in 30 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) CYCLOMETHICONE (UNII: NMQ347994Z) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) ERYTHRITOL (UNII: RA96B954X6) XYLITOL (UNII: VCQ006KQ1E) BARIUM SULFATE (UNII: 25BB7EKE2E) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) ROSA ROXBURGHII FRUIT (UNII: CVT1AA87FF) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) ALUMINUM DISTEARATE (UNII: 7P1HP1B9UI) EDETATE DISODIUM (UNII: 7FLD91C86K) ALUMINUM OXIDE (UNII: LMI26O6933) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) DIPROPYLENE GLYCOL (UNII: E107L85C40) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ALCOHOL (UNII: 3K9958V90M) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICA (UNII: V8A1AW0880) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-204-60 1 in 1 CARTON 09/01/2014 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 09/01/2014 Labeler - SHISEIDO AMERICAS CORPORATION (193691821) Establishment Name Address ID/FEI Business Operations SHISEIDO AMERICA INC. 782677132 manufacture(58411-193, 58411-194, 58411-195, 58411-196, 58411-197, 58411-198, 58411-199, 58411-200, 58411-201, 58411-202, 58411-203, 58411-204) , analysis(58411-193, 58411-194, 58411-195, 58411-196, 58411-197, 58411-198, 58411-199, 58411-200, 58411-201, 58411-202, 58411-203, 58411-204)