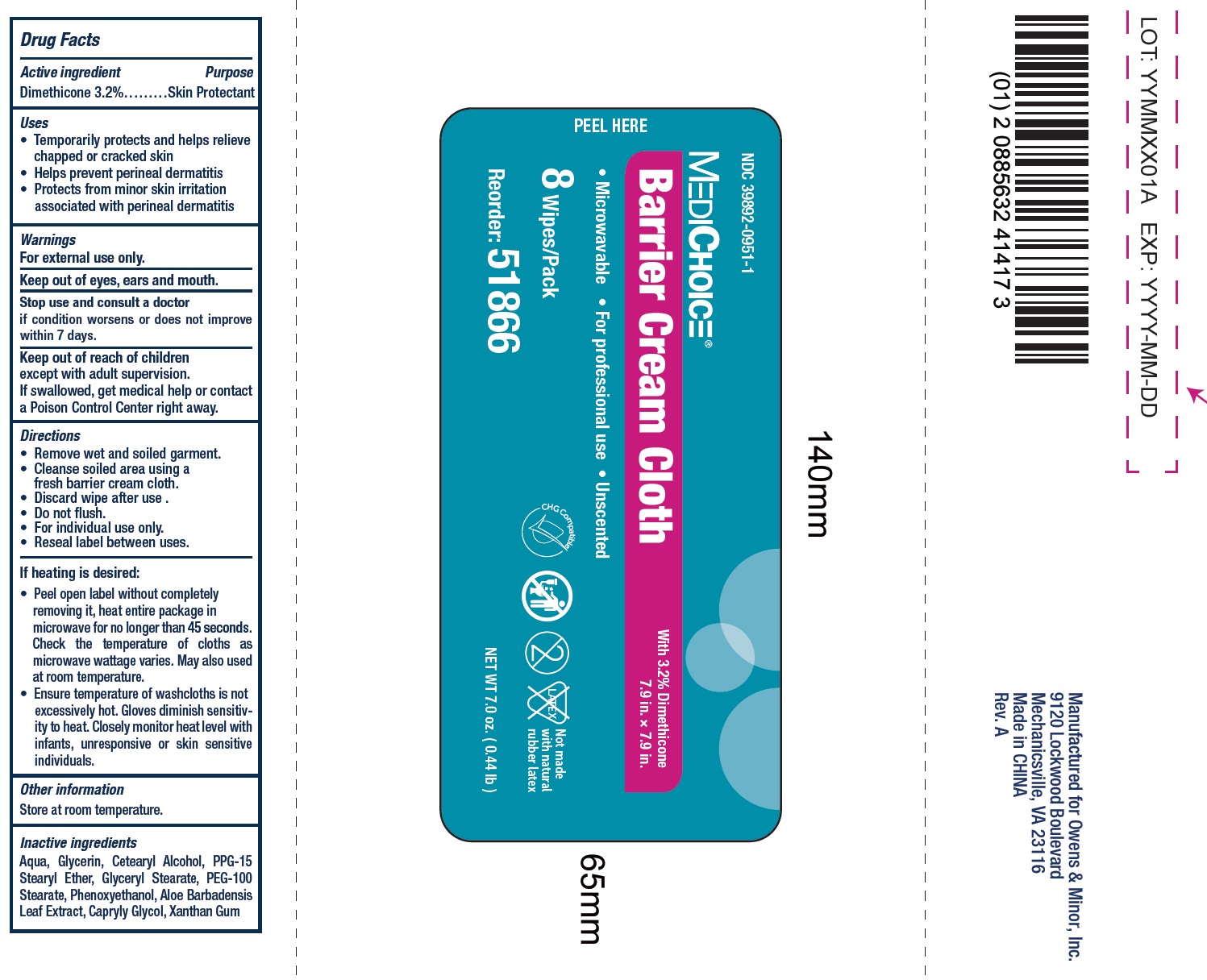
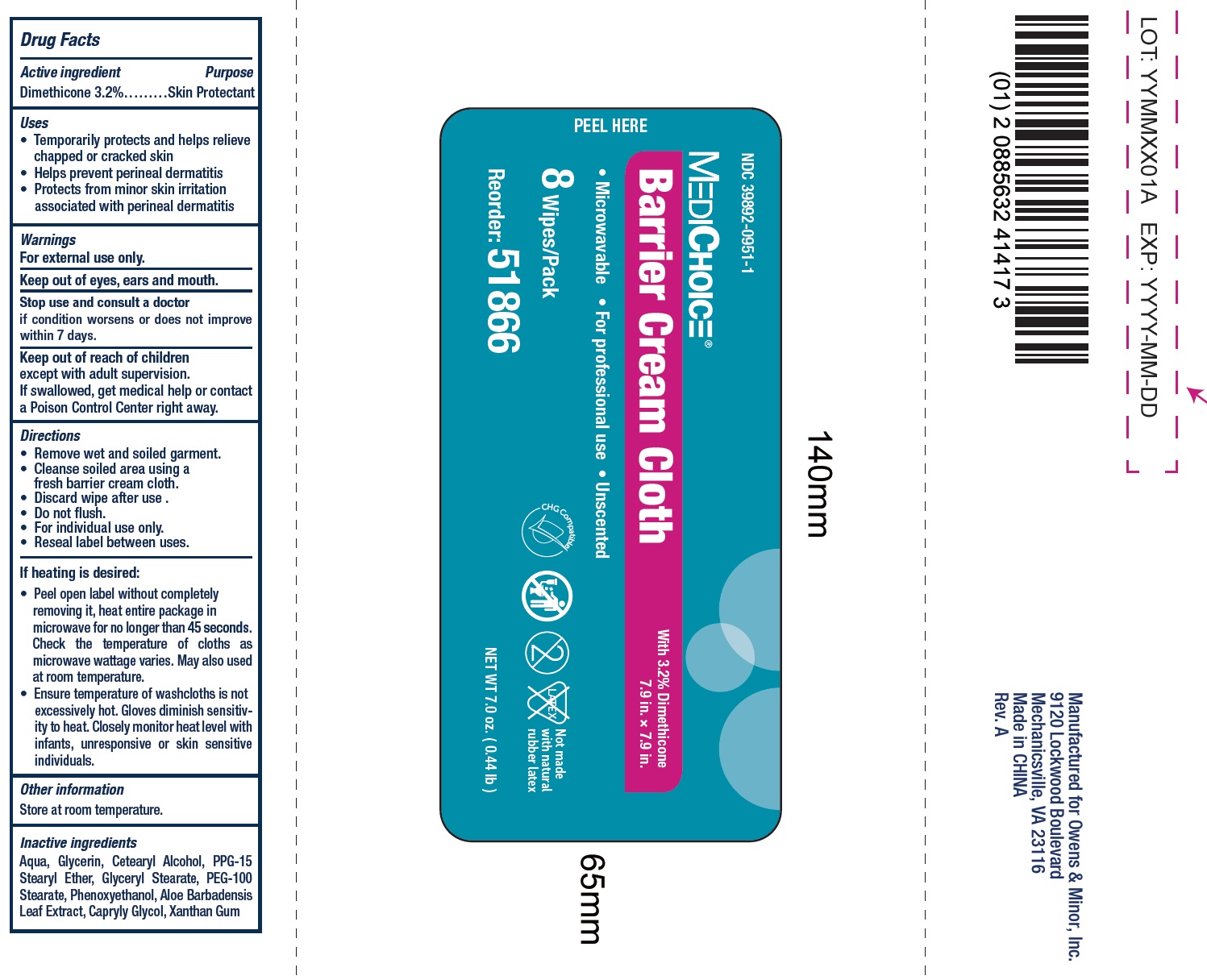
Label: MEDICHOICE BARRIER- dimethicone cloth
- NDC Code(s): 39892-0951-1, 39892-0951-2
- Packager: Owens & Minor Distribution, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Uses
- Warnings
- Directions
-
If heating is desired:
• Peel open label without completely removing it, heat entire package in microwave for no longer than 45 seconds. Check the temperature of cloths as microwave wattage varies. May also used at room temperature. • Ensure temperature of washcloths is not excessively hot. Gloves diminish sensitivity to heat. Closely monitor heat level with infants, unresponsive or skin sensitive individuals.
- Inactive ingredients
- Package Labeling:
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
MEDICHOICE BARRIER
dimethicone clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:39892-0951 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 32 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) PPG-15 STEARYL ETHER (UNII: 1II18XLS1L) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) PHENOXYETHANOL (UNII: HIE492ZZ3T) ALOE VERA LEAF (UNII: ZY81Z83H0X) CAPRYLYL GLYCOL (UNII: 00YIU5438U) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:39892-0951-1 8 in 1 PACKAGE 01/25/2024 1 20 g in 1 PATCH; Type 0: Not a Combination Product 2 NDC:39892-0951-2 8 in 1 CASE 01/25/2024 2 30 in 1 PACKAGE 2 20 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug OKM016 01/25/2024 Labeler - Owens & Minor Distribution, Inc. (847412269)