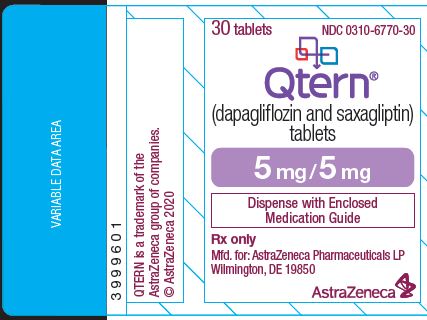
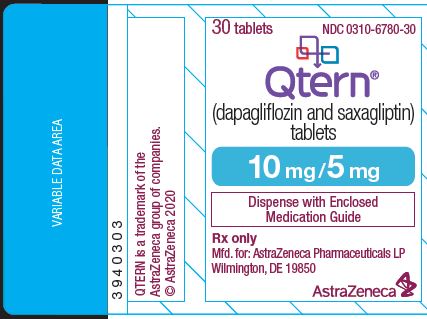
Label: QTERN- dapagliflozin and saxagliptin tablet, film coated
- NDC Code(s): 0310-6770-30, 0310-6770-95, 0310-6780-30, 0310-6780-95
- Packager: AstraZeneca Pharmaceuticals LP
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use QTERN safely and effectively. See full prescribing information for QTERN.
QTERN® (dapagliflozin and saxagliptin) tablets, for oral use
Initial U.S. Approval: 2017INDICATIONS AND USAGE
QTERN is a combination of dapagliflozin, a sodium-glucose cotransporter 2 (SGLT2) inhibitor and saxagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. (1)
Limitations of Use:
Not recommended for use to improve glycemic control in patients with type 1 diabetes mellitus. (1)
DOSAGE AND ADMINISTRATION
- •
- Assess renal function before initiation of therapy and periodically thereafter. (2.1)
- •
- Take orally, once daily in the morning with or without food. (2.2)
- •
- For patients not already taking dapagliflozin, the recommended starting dose of QTERN is a 5 mg dapagliflozin/5 mg saxagliptin tablet once daily. (2.2)
- •
- In patients tolerating 5 mg dapagliflozin and 5 mg saxagliptin once daily who require additional glycemic control, the QTERN dose can be increased to 10 mg dapagliflozin/5 mg saxagliptin tablet once daily. (2.2)
- •
- Swallow tablet whole. Do not crush, cut or chew. (2.2)
- •
- Withhold QTERN for at least 3 days, if possible, prior to major surgery or procedures associated with prolonged fasting. (2.5)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- •
- Diabetic Ketoacidosis in Patients with Type 1 Diabetes Mellitus and Other Ketoacidosis: Consider ketone monitoring in patients at risk for ketoacidosis, as indicated. Assess for ketoacidosis regardless of presenting blood glucose levels and discontinue QTERN if ketoacidosis is suspected. Monitor patients for resolution of ketoacidosis before restarting. (5.1)
- •
- Pancreatitis: If pancreatitis is suspected, promptly discontinue QTERN. (5.2)
- •
- Heart Failure: Consider risks and benefits of QTERN in patients who have known risk factors for heart failure. Monitor patients for signs and symptoms. (5.3)
- •
- Volume Depletion: Before initiating QTERN, assess volume status and renal function in the elderly, patients with renal impairment or low systolic blood pressure, and in patients on diuretics. Monitor for signs and symptoms during therapy. (5.4)
- •
- Urosepsis and Pyelonephritis: Evaluate for signs and symptoms of urinary tract infections and treat promptly, if indicated. (5.5)
- •
- Hypoglycemia: Consider lowering the dose of insulin secretagogue or insulin to reduce the risk of hypoglycemia when initiating QTERN. (5.6)
- •
- Necrotizing Fasciitis of the Perineum (Fournier’s Gangrene): Serious, life-threatening cases have occurred in both females and males. Assess patients presenting with pain or tenderness, erythema, or swelling in the genital or perineal area, along with fever or malaise. If suspected, institute prompt treatment. (5.7)
- •
- Hypersensitivity Reactions: There have been postmarketing reports of serious hypersensitivity reactions in patients treated with saxagliptin, such as anaphylaxis, angioedema, and exfoliative skin conditions. Promptly discontinue QTERN, assess for other potential causes, institute appropriate monitoring and treatment, and initiate alternative treatment for diabetes. (5.8)
- •
- Genital Mycotic Infections: Monitor and treat if indicated. (5.9)
- •
- Arthralgia: Severe and disabling arthralgia has been reported in patients taking DPP-4 inhibitors. Consider as a possible cause for severe joint pain and discontinue QTERN if appropriate. (5.10)
- •
- Bullous Pemphigoid: There have been postmarketing reports of bullous pemphigoid requiring hospitalization in patients taking DPP-4 inhibitors. Tell patients to report development of blisters or erosions. If bullous pemphigoid is suspected, discontinue QTERN. (5.11)
ADVERSE REACTIONS
Adverse reactions reported in ≥5% of subjects treated with dapagliflozin and saxagliptin were upper respiratory tract infection, urinary tract infection, and dyslipidemia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact AstraZeneca at 1-800-236-9933 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
- •
- Pregnancy: Advise females of the potential risk to a fetus especially during the second and third trimesters. (8.1)
- •
- Lactation: Not recommended when breastfeeding. (8.2)
- •
- Geriatrics: Higher incidence of adverse reactions related to hypotension. (8.5)
- •
- Renal Impairment: Higher incidence of adverse reactions related to volume depletion. (8.6)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 11/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Prior to Initiation of QTERN
2.2 Dosage
2.3 Patients with Renal Impairment
2.4 Use with Strong CYP3A4/5 Inhibitors
2.5 Temporary Interruption for Surgery
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Diabetic Ketoacidosis in Patients with Type 1 Diabetes Mellitus and Other Ketoacidosis
5.2 Pancreatitis
5.3 Heart Failure
5.4 Volume Depletion
5.5 Urosepsis and Pyelonephritis
5.6 Hypoglycemia with Concomitant Use of Insulin or Insulin Secretagogues
5.7 Necrotizing Fasciitis of the Perineum (Fournier’s Gangrene)
5.8 Hypersensitivity Reactions
5.9 Genital Mycotic Infections
5.10 Severe and Disabling Arthralgia
5.11 Bullous Pemphigoid
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Add-on Therapy with Dapagliflozin plus Saxagliptin in Patients on Metformin
14.2 Add-on Therapy with Saxagliptin in Patients on Dapagliflozin plus Metformin
14.3 Cardiovascular Safety Trial
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
QTERN is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
Limitations of Use
QTERN is not recommended for use to improve glycemic control in patients with type 1 diabetes mellitus [see WARNINGS AND PRECAUTIONS (5.1)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Prior to Initiation of QTERN
Assess renal function prior to initiation of QTERN therapy and periodically thereafter [see WARNINGS AND PRECAUTIONS (5.4)].
Assess volume status. In patients with volume depletion, correct this condition before initiating QTERN [see WARNINGS AND PRECAUTIONS (5.4) and USE IN SPECIFIC POPULATIONS (8.5, 8.6)].
2.2 Dosage
For patients not already taking dapagliflozin, the recommended starting dose of QTERN is a 5 mg dapagliflozin/5 mg saxagliptin tablet taken orally once daily in the morning with or without food.
In patients tolerating 5 mg dapagliflozin and 5 mg saxagliptin once daily who require additional glycemic control, the QTERN dose can be increased to 10 mg dapagliflozin/5 mg saxagliptin tablet once daily in the morning with or without food.
Swallow whole. Do not crush, cut or chew QTERN tablets.
2.3 Patients with Renal Impairment
No dose adjustment is needed in patients with an estimated glomerular filtration rate (eGFR) greater than or equal to 45 mL/min/1.73 m2.
QTERN is contraindicated in patients with an eGFR less than 45 mL/min/1.73 m2 [see CONTRAINDICATIONS (4) and USE IN SPECIFIC POPULATIONS (8.6)].
2.4 Use with Strong CYP3A4/5 Inhibitors
Do not coadminister QTERN with strong cytochrome P450 3A4/5 inhibitors (e.g., ketoconazole, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, ritonavir, saquinavir, and telithromycin) [see DRUG INTERACTIONS (7)].
2.5 Temporary Interruption for Surgery
Withhold QTERN for at least 3 days, if possible, prior to major surgery or procedures associated with prolonged fasting. Resume QTERN when the patient is clinically stable and has resumed oral intake [see WARNINGS AND PRECAUTIONS (5.1) and CLINICAL PHARMACOLOGY (12.2)].
-
3 DOSAGE FORMS AND STRENGTHS
QTERN (dapagliflozin and saxagliptin) tablets are available as follows:
Table 1: Dosage Forms and Strengths for QTERN Dapagliflozin
StrengthSaxagliptin
StrengthColor/Shape Tablet Markings 5 mg
5 mg
Light purple to reddish purple, biconvex, round, film‑coated tablet
“1120” printed on both sides, in blue ink
10 mg
5 mg
Light brown to brown, biconvex, round, film‑coated tablet
“1122” printed on both sides, in blue ink
-
4 CONTRAINDICATIONS
QTERN is contraindicated in patients with:
- •
- History of a serious hypersensitivity reaction to dapagliflozin or to saxagliptin, including anaphylactic reactions, angioedema or exfoliative skin conditions [see WARNINGS AND PRECAUTIONS (5.8) and ADVERSE REACTIONS (6.2)].
- •
- Moderate to severe renal impairment (eGFR less than 45 mL/min/1.73 m2), end-stage renal disease (ESRD), or patients on dialysis [see USE IN SPECIFIC POPULATIONS (8.6)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Diabetic Ketoacidosis in Patients with Type 1 Diabetes Mellitus and Other Ketoacidosis
In patients with type 1 diabetes mellitus, dapagliflozin, a component of QTERN, significantly increases the risk of diabetic ketoacidosis, a life-threatening event, beyond the background rate. In placebo-controlled trials of patients with type 1 diabetes mellitus, the risk of ketoacidosis was markedly increased in patients who received sodium-glucose cotransporter 2 (SGLT2) inhibitors compared to patients who received placebo. QTERN is not indicated for glycemic control in patients with type 1 diabetes mellitus.
Type 2 diabetes mellitus and pancreatic disorders (e.g., history of pancreatitis or pancreatic surgery) are also risk factors for ketoacidosis. There have been postmarketing reports of fatal events of ketoacidosis in patients with type 2 diabetes mellitus using SGLT2 inhibitors, including dapagliflozin.
Precipitating conditions for diabetic ketoacidosis or other ketoacidosis include under-insulinization due to insulin dose reduction or missed insulin doses, acute febrile illness, reduced caloric intake, ketogenic diet, surgery, volume depletion, and alcohol abuse.
Signs and symptoms are consistent with dehydration and severe metabolic acidosis and include nausea, vomiting, abdominal pain, generalized malaise, and shortness of breath. Blood glucose levels at presentation may be below those typically expected for diabetic ketoacidosis (e.g., less than 250 mg/dL). Ketoacidosis and glucosuria may persist longer than typically expected. Urinary glucose excretion persists for 3 days after discontinuing QTERN [see CLINICAL PHARMACOLOGY (12.2)]; however, there have been postmarketing reports of ketoacidosis and/or glucosuria lasting greater than 6 days and some up to 2 weeks after discontinuation of SGLT2 inhibitors.
Consider ketone monitoring in patients at risk for ketoacidosis if indicated by the clinical situation. Assess for ketoacidosis regardless of presenting blood glucose levels in patients who present with signs and symptoms consistent with severe metabolic acidosis. If ketoacidosis is suspected, discontinue QTERN, promptly evaluate, and treat ketoacidosis, if confirmed. Monitor patients for resolution of ketoacidosis before restarting QTERN.
Withhold QTERN, if possible, in temporary clinical situations that could predispose patients to ketoacidosis. Resume QTERN when the patient is clinically stable and has resumed oral intake [see DOSAGE AND ADMINISTRATION (2.5)].
Educate all patients on the signs and symptoms of ketoacidosis and instruct patients to discontinue QTERN and seek medical attention immediately if signs and symptoms occur.
5.2 Pancreatitis
There have been postmarketing reports of acute pancreatitis in patients taking saxagliptin. In a cardiovascular outcomes trial enrolling participants with established atherosclerotic cardiovascular disease (ASCVD) or multiple risk factors for ASCVD (SAVOR trial), cases of definite acute pancreatitis were confirmed in 17 of 8240 (0.2%) patients receiving saxagliptin compared to 9 of 8173 (0.1%) receiving placebo. Pre-existing risk factors for pancreatitis were identified in 88% (15/17) of those patients receiving saxagliptin and in 100% (9/9) of those patients receiving placebo.
After initiation of QTERN, observe patients for signs and symptoms of pancreatitis. If pancreatitis is suspected, promptly discontinue QTERN and initiate appropriate management. It is unknown whether patients with a history of pancreatitis are at increased risk for the development of pancreatitis while using QTERN.
5.3 Heart Failure
In a cardiovascular outcomes trial enrolling participants with established ASCVD or multiple risk factors for ASCVD (SAVOR trial), more patients randomized to saxagliptin (289/8280, 3.5%) were hospitalized for heart failure compared to patients randomized to placebo (228/8212, 2.8%). In a time-to-first-event analysis the risk of hospitalization for heart failure was higher in the saxagliptin group (estimated Hazard Ratio: 1.27; 95% CI: 1.07, 1.51). Subjects with a prior history of heart failure and subjects with renal impairment had a higher risk for hospitalization for heart failure, irrespective of treatment assignment.
Consider the risks and benefits of QTERN prior to initiating treatment in patients at a higher risk of heart failure. Observe patients for signs and symptoms of heart failure during therapy. Advise patients of the characteristic symptoms of heart failure and to immediately report such symptoms. If heart failure develops, evaluate and manage according to current standards of care and consider discontinuation of QTERN.
5.4 Volume Depletion
Dapagliflozin can cause intravascular volume depletion which may sometimes manifest as symptomatic hypotension or acute transient changes in creatinine. There have been postmarketing reports of acute kidney injury, some requiring hospitalization and dialysis, in patients with type 2 diabetes mellitus receiving SGLT2 inhibitors, including dapagliflozin. Patients with impaired renal function (eGFR <60 mL/min/1.73 m2), elderly patients, or patients on loop diuretics may be at increased risk for volume depletion or hypotension. Before initiating QTERN in patients with one or more of these characteristics, assess volume status and renal function. QTERN is contraindicated in patients with an eGFR <45 mL/min/1.73 m2. Monitor for signs and symptoms of hypotension, and renal function after initiating therapy.
5.5 Urosepsis and Pyelonephritis
Serious urinary tract infections including urosepsis and pyelonephritis requiring hospitalization have been reported in patients receiving SGLT2 inhibitors, including dapagliflozin. Treatment with SGLT2 inhibitors increases the risk for urinary tract infections. Evaluate patients for signs and symptoms of urinary tract infections and treat promptly, if indicated [see ADVERSE REACTIONS (6.2)].
5.6 Hypoglycemia with Concomitant Use of Insulin or Insulin Secretagogues
Insulin and insulin secretagogues, such as sulfonylureas, are known to cause hypoglycemia. Both dapagliflozin and saxagliptin can individually increase the risk of hypoglycemia when combined with insulin or an insulin secretagogue. Therefore, a lower dose of insulin or insulin secretagogue may be required to reduce the risk of hypoglycemia when these agents are used in combination with QTERN [see ADVERSE REACTIONS (6.1)].
5.7 Necrotizing Fasciitis of the Perineum (Fournier’s Gangrene)
Reports of necrotizing fasciitis of the perineum (Fournier’s Gangrene), a rare but serious and life-threatening necrotizing infection requiring urgent surgical intervention, have been identified in postmarketing surveillance in patients with diabetes mellitus receiving SGLT2 inhibitors, including dapagliflozin. Cases have been reported in both females and males. Serious outcomes have included hospitalization, multiple surgeries, and death.
Patients treated with QTERN presenting with pain or tenderness, erythema, or swelling in the genital or perineal area, along with fever or malaise, should be assessed for necrotizing fasciitis. If suspected, start treatment immediately with broad-spectrum antibiotics and, if necessary, surgical debridement. Discontinue QTERN, closely monitor blood glucose levels, and provide appropriate alternative therapy for glycemic control.
5.8 Hypersensitivity Reactions
There have been postmarketing reports of serious hypersensitivity reactions in patients treated with saxagliptin. These reactions include anaphylactic reactions, angioedema, and exfoliative skin conditions. Onset of these reactions occurred within the first 3 months after initiation of treatment with saxagliptin, with some reports occurring after the first dose. If a serious hypersensitivity reaction is suspected, discontinue QTERN, treat per standard of care, and monitor until signs and symptoms are resolved. Assess for other potential causes for the event. Institute alternative treatment for diabetes.
Use caution in a patient with a history of angioedema to another dipeptidyl peptidase-4 (DPP-4) inhibitor because it is unknown whether such patients will be predisposed to angioedema with saxagliptin.
5.9 Genital Mycotic Infections
Dapagliflozin increases the risks of genital mycotic infections. Patients with a history of genital mycotic infections were more likely to develop genital mycotic infections [see ADVERSE REACTIONS (6.1)]. Monitor and treat appropriately.
5.10 Severe and Disabling Arthralgia
There have been postmarketing reports of severe and disabling arthralgia in patients taking DPP-4 inhibitors. The time to onset of symptoms following initiation of drug therapy varied from one day to years. Patients experienced relief of symptoms upon discontinuation of the medication. A subset of patients experienced a recurrence of symptoms restarting the same drug or a different DPP-4 inhibitor. Consider DPP-4 inhibitors as a possible cause for severe joint pain and discontinue drug if appropriate [see ADVERSE REACTIONS (6)].
5.11 Bullous Pemphigoid
Postmarketing cases of bullous pemphigoid requiring hospitalization have been reported with DPP-4 inhibitor use. In reported cases, patients typically recovered with topical or systemic immunosuppressive treatment and discontinuation of the DPP-4 inhibitor. Tell patients to report development of blisters or erosions while receiving QTERN. If bullous pemphigoid is suspected, QTERN should be discontinued and referral to a dermatologist should be considered for diagnosis and appropriate treatment.
-
6 ADVERSE REACTIONS
The following important adverse reactions are described below or elsewhere in the labeling:
- •
- Diabetic Ketoacidosis in Patients with Type 1 Diabetes Mellitus and Other Ketoacidosis [see WARNINGS AND PRECAUTIONS (5.1)]
- •
- Pancreatitis [see WARNINGS AND PRECAUTIONS (5.2)]
- •
- Heart Failure [see WARNINGS AND PRECAUTIONS (5.3)]
- •
- Volume Depletion [see WARNINGS AND PRECAUTIONS (5.4)]
- •
- Urosepsis and Pyelonephritis [see WARNINGS AND PRECAUTIONS (5.5)]
- •
- Hypoglycemia with Concomitant Use of Insulin or Insulin Secretagogues [see WARNINGS AND PRECAUTIONS (5.6)]
- •
- Necrotizing Fasciitis of the Perineum (Fournier’s Gangrene) [see WARNINGS AND PRECAUTIONS (5.7)]
- •
- Hypersensitivity Reactions [see WARNINGS AND PRECAUTIONS (5.8)]
- •
- Genital Mycotic Infections [see WARNINGS AND PRECAUTIONS (5.9)]
- •
- Severe and Disabling Arthralgia [see WARNINGS AND PRECAUTIONS (5.10)]
- •
- Bullous Pemphigoid [see WARNINGS AND PRECAUTIONS (5.11)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of combined use of 10 mg dapagliflozin and 5 mg saxagliptin has been evaluated in adult subjects with type 2 diabetes mellitus in a pooled safety analysis of three phase 3 active/placebo-controlled clinical trials with a median exposure of 51 weeks. The pooled safety analysis included a total of 1169 adults: 492 patients in the combination of saxagliptin and dapagliflozin plus metformin group, 341 patients in the dapagliflozin plus metformin group, 336 patients in the saxagliptin plus metformin group. The mean age of these subjects was 54 years, 0.8% were 75 years or older and 53.7% were female. The population was 80.9% White, 8.3% Black or African American, 3.7% Asian, and 6.6% Other race. At baseline the population had diabetes for an average of 7.5 years and a mean HbA1c of 8.4%. The mean eGFR at baseline was 94.4 mL/min/1.73 m2.
The common adverse reactions were based on the pooled analyses of these studies as shown in Table 2.
Table 2: Adverse Reactions Reported in ≥2% of Subjects Treated with 10 mg Dapagliflozin and 5 mg Saxagliptin plus Metformin (≥1500 mg) - *
- Adverse reactions that are medically related were grouped to a single preferred term.
Adverse Reaction
Preferred Term*
Frequency %
Upper respiratory tract infection*
13.6
Urinary tract infection*
5.7
Dyslipidemia*
5.1
Headache
4.3
Diarrhea
3.7
Back pain
3.3
Genital infection*
3.0
Arthralgia
2.4
Additionally, adverse reactions reported in <5% and ≥2% from the dapagliflozin development program and ≥1% more frequently compared to placebo included increased urination and discomfort with urination.
Hypoglycemia
In the pooled analysis, the incidences of hypoglycemia (defined as a blood glucose <54 mg/dL regardless of the presence or absence of symptoms) and severe hypoglycemia (event requiring assistance due to neuroglycopenia, characterized by altered mental and/or physical status) was 1% and 0.2%, respectively.
Genital Mycotic Infections
Genital mycotic infections were reported in 15 subjects (3%) treated with QTERN. Reported adverse reactions by frequency included vulvovaginal mycotic infection, balanoposthitis, genital fungal infection, vaginal infection, and vulvovaginitis. The majority of subjects (84.2%) who experienced genital infection adverse reactions were females.
Urinary Tract Infections
Urinary tract infections were reported in 28 subjects (5.7%) treated with QTERN. Reported adverse reactions by frequency included urinary tract infection, Escherichia urinary tract infection, prostatitis, and pyelonephritis. The majority of subjects (80.6%) who experienced urinary tract infection adverse reactions were females.
Volume Depletion
Dapagliflozin causes an osmotic diuresis, which may lead to a reduction in intravascular volume. Events related to volume depletion (hypotension, dehydration, and hypovolemia) were reported in 2 subjects (0.4%) treated with QTERN plus metformin.
Impairment of Renal Function
Adverse reactions related to decreased renal function were reported in 10 subjects (2.0%) treated with QTERN plus metformin. The reported adverse reactions included decreased glomerular filtration rate, renal impairment, increased blood creatinine, acute renal failure, and decreased urine output. None of the adverse reactions were reported as serious and all but one was mild to moderate in intensity. Three subjects discontinued due to decreased eGFR. Subjects with AEs of renal impairment had lower mean eGFR values at baseline of 64.4 mL/min/1.73 m2 compared to 94.4 mL/min/1.73 m2 in overall population treated with QTERN.
Ketoacidosis
Dapagliflozin
In the cardiovascular outcome study with dapagliflozin in patients with type 2 diabetes mellitus, events of diabetic ketoacidosis (DKA) were reported in 27 out of 8574 patients in the dapagliflozin-treated group and in 12 out of 8569 patients in the placebo group. The events were evenly distributed over the study period.
Laboratory Tests
Increases in Serum Creatinine and Decreases in eGFR
Dapagliflozin
Initiation of SGLT2 inhibitors, including dapagliflozin causes a small increase in serum creatinine and decrease in eGFR. These changes in serum creatinine and eGFR generally occur within two weeks of starting therapy and then stabilize regardless of baseline kidney function. Changes that do not fit this pattern should prompt further evaluation to exclude the possibility of acute kidney injury. In two studies that included patients with type 2 diabetes mellitus with moderate renal impairment, the acute effect on eGFR reversed after treatment discontinuation, suggesting acute hemodynamic changes may play a role in the renal function changes observed with dapagliflozin.
Decrease in Lymphocyte Counts
Saxagliptin
A dose-related mean decrease in absolute lymphocyte count has been observed with saxagliptin. In a pool of 5 placebo-controlled studies, a mean decrease in absolute lymphocyte count of approximately 100 cells/microL relative to placebo was observed. The proportion of patients who were reported to have a lymphocyte count ≤750 cells/microL was 0.5%, 1.5%, and 0.4% in the 2.5 mg, 5 mg saxagliptin and placebo groups, respectively.
The clinical significance of this decrease in lymphocyte count relative to placebo is not known. The effect of saxagliptin on lymphocyte counts in patients with lymphocyte abnormalities (e.g., human immunodeficiency virus) is unknown.
Increase in Hematocrit
Dapagliflozin
In a pool of 13 placebo-controlled studies with dapagliflozin, increases from baseline in mean hematocrit values were observed in dapagliflozin-treated patients starting at Week 1 and continuing up to Week 16, when the maximum mean difference from baseline was observed. At Week 24, the mean changes from baseline in hematocrit were -0.33% in the placebo group and 2.30% in the 10 mg dapagliflozin group. By Week 24, hematocrit values >55% were reported in 0.4% of placebo-treated patients and 1.3% of 10 mg dapagliflozin-treated patients.
Increase in Low-Density Lipoprotein Cholesterol
Patients treated with QTERN demonstrated a mean percent increase from baseline LDL-cholesterol (ranging from 2.1 to 6.9%).
Elevations in Creatine Kinase
An imbalance in the number of subjects who experienced serum creatine kinase (CK) elevations >10x the upper limit of normal (a marker of muscle injury/necrosis) was observed in 5 subjects (1%) treated with QTERN. The elevations were transient. Rhabdomyolysis was reported for one of those subjects for which no obvious cause was identified.
Decrease in Serum Bicarbonate
In a study of concomitant therapy of 10 mg dapagliflozin with exenatide extended-release (on a background of metformin), four patients (1.7%) on concomitant therapy had a serum bicarbonate value of less than or equal to 13 mEq/L compared to one each (0.4%) in the dapagliflozin and exenatide-extended release treatment groups.
6.2 Postmarketing Experience
Additional adverse reactions have been identified during post-approval use of dapagliflozin and saxagliptin. Because the following reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Dapagliflozin
Infections: Necrotizing fasciitis of the perineum (Fournier’s Gangrene), urosepsis and pyelonephritis
Metabolism and Nutrition Disorders: Ketoacidosis
Renal and Urinary Disorders: Acute kidney injury
Skin and Subcutaneous Tissue Disorders: Rash
Saxagliptin
Gastrointestinal Disorders: Pancreatitis
Immune System Disorders: Hypersensitivity reactions including anaphylaxis, angioedema, and exfoliative skin conditions
Musculoskeletal and Connective Tissue Disorders: Rhabdomyolysis, severe and disabling arthralgia
Skin and Subcutaneous Tissue Disorders: Bullous pemphigoid
-
7 DRUG INTERACTIONS
Table 3: Clinically Relevant Interactions with QTERN Strong Inhibitors of CYP3A4/5 Enzymes
Clinical Impact
Ketoconazole significantly increased saxagliptin exposure. Similar significant increases in plasma concentrations of saxagliptin are anticipated with other strong CYP3A4/5 inhibitors (e.g., atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, ritonavir, saquinavir, and telithromycin).
Intervention
Do not coadminister QTERN with strong cytochrome P450 3A4/5 inhibitors [see DOSAGE AND ADMINISTRATION (2.4) and CLINICAL PHARMACOLOGY (12.3)].
Insulin or Insulin Secretagogues
Clinical Impact
The risk of hypoglycemia may be increased when QTERN is used concomitantly with insulin or insulin secretagogues (e.g., sulfonylurea) [see WARNINGS AND PRECUATIONS (5.6)].
Intervention
Concomitant use may require lower doses of insulin or the insulin secretagogue to reduce the risk of hypoglycemia.
Lithium
Clinical Impact
Concomitant use of an SGLT2 inhibitor with lithium may decrease serum lithium concentrations.
Intervention
Monitor serum lithium concentration more frequently during QTERN initiation and dosage changes.
Positive Urine Glucose Test
Clinical Impact
SGLT2 inhibitors increase urinary glucose excretion and will lead to positive urine glucose tests.
Intervention
Monitoring glycemic control with urine glucose tests is not recommended in patients taking SGLT2 inhibitors. Use alternative methods to monitor glycemic control.
Interference with 1,5‑anhydroglucitol (1,5‑AG) Assay
Clinical Impact
Measurements of 1,5‑AG are unreliable in assessing glycemic control in patients taking SGLT2 inhibitors.
Intervention
Monitoring glycemic control with 1,5‑AG assay is not recommended. Use alternative methods to monitor glycemic control.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on animal data showing adverse renal effects from dapagliflozin, QTERN is not recommended during the second and third trimesters of pregnancy.
The limited available data with QTERN or its components (dapagliflozin and saxagliptin) in pregnant women are not sufficient to determine a drug-associated risk for major birth defects or miscarriage. There are risks to the mother and fetus associated with poorly controlled diabetes in pregnancy (see Clinical Considerations).
In animal studies, adverse renal pelvic and tubular dilatations, that were not fully reversible, were observed in rats when dapagliflozin (a component of QTERN) was administered during a period of renal development corresponding to the late second and third trimesters of human pregnancy, at all doses tested; the lowest of which provided an exposure 15-times the 10 mg clinical dose (see Data).
No adverse developmental effects were observed when saxagliptin was administered to pregnant rats and rabbits (see Data).
The estimated background risk of major birth defects is 6 to 10% in women with pre-gestational diabetes with an HbA1c greater than 7% and has been reported to be as high as 20 to 25% in women with an HbA1c greater than 10%. The estimated background risk of miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Disease-associated maternal and/or embryofetal risk
Poorly controlled diabetes in pregnancy increases the maternal risk for diabetic ketoacidosis, preeclampsia, spontaneous abortions, preterm delivery and delivery complications. Poorly controlled diabetes increases the fetal risk for major birth defects, stillbirth, and macrosomia-related morbidity.
Animal Data
Dapagliflozin
Dapagliflozin dosed directly to juvenile rats from postnatal day (PND) 21 until PND 90 at doses of 1, 15, or 75 mg/kg/day, increased kidney weights and increased the incidence of renal pelvic and tubular dilatations at all dose levels. Exposure at the lowest dose was 15-times the 10 mg clinical dose (based on AUC). The renal pelvic and tubular dilatations observed in juvenile animals did not fully reverse within a 1-month recovery period.
In a prenatal and postnatal development study, dapagliflozin was administered to maternal rats from gestation Day 6 through lactation Day 21 at doses of 1, 15, or 75 mg/kg/day, and pups were indirectly exposed in utero and throughout lactation. Increased incidence or severity of renal pelvic dilatation was observed in 21 day-old pup offspring of treated dams at 75 mg/kg/day (maternal and pup dapagliflozin exposures were 1415-times and 137-times, respectively, the human values at the 10 mg clinical dose, based on AUC). Dose-related reductions in pup body weights were observed at greater than or equal to 29-times the 10 mg clinical dose (based on AUC). No adverse effects on developmental endpoints were noted at 1 mg/kg/day, (19-times the 10 mg clinical dose, based on AUC). These outcomes occurred with drug exposure during periods of renal development in rats that corresponds to the late second and third trimester of human development.
In embryofetal development studies in rats and rabbits, dapagliflozin was administered throughout organogenesis, corresponding to the first trimester of human pregnancy. In rats, dapagliflozin was neither embryolethal nor teratogenic at doses up to 75 mg/kg/day (1441-times the 10 mg clinical dose, based on AUC). Dose-related effects on the rat fetus (structural abnormalities and reduced body weight) occurred only at higher dosages, equal to or greater than 150 mg/kg (more than 2344-times the 10 mg clinical dose, based on AUC), which were associated with maternal toxicity. No developmental toxicities were observed in rabbits at doses up to 180 mg/kg/day (1191-times the 10 mg clinical dose, based on AUC).
Saxagliptin
In embryofetal development studies, saxagliptin was administered to pregnant rats and rabbits during the period of organogenesis, corresponding to the first trimester of human pregnancy. No adverse developmental effects were observed in either species at exposures 1503- and 152-times the 5 mg clinical dose in rats and rabbits, respectively, based on AUC. Saxagliptin crosses the placenta into the fetus following dosing in pregnant rats.
In a prenatal and postnatal development study, no adverse developmental effects were observed in maternal rats administered saxagliptin from gestation day 6 through lactation day 21 at exposures up to 470-times the 5 mg clinical dose, based on AUC.
8.2 Lactation
Risk Summary
There is no information regarding the presence of QTERN or its components (dapagliflozin and saxagliptin) in human milk, the effects on the breastfed infant, or the effects on milk production.
Dapagliflozin and saxagliptin are present in the milk of lactating rats (see Data). However, due to species-specific differences in lactation physiology, the clinical relevance of these data is not clear. Since human kidney maturation occurs in utero and during the first 2 years of life when lactational exposure may occur, there may be risk to the developing human kidney. Because of the potential for serious adverse reactions in a breastfed infant, advise women that use of QTERN is not recommended while breastfeeding.
Dapagliflozin
Dapagliflozin was present at a milk/plasma ratio of 0.49, indicating that dapagliflozin and its metabolites are transferred into milk at a concentration that is approximately 50% of that in maternal plasma. Juvenile rats directly exposed to dapagliflozin showed a risk to the developing kidney (renal pelvic and tubular dilatations) during maturation.
Saxagliptin
Saxagliptin is secreted in the milk of lactating rats at approximately a 1:1 ratio with plasma drug concentrations.
8.4 Pediatric Use
Safety and effectiveness of QTERN in pediatric patients under 18 years of age have not been established.
8.5 Geriatric Use
Because elderly patients are more likely to have decreased renal function, care should be taken when using QTERN in the elderly based on renal function [see DOSAGE AND ADMINISTRATION (2.3)].
Dapagliflozin
A total of 1424 (24%) of the 5936 dapagliflozin-treated patients were 65 years and older and 207 (3.5%) patients were 75 years and older in a pool of 21 double-blind, controlled, clinical studies assessing the efficacy of dapagliflozin in improving glycemic control. After controlling for level of renal function (eGFR), in clinical studies with dapagliflozin, efficacy was similar for patients under age 65 years and those 65 years and older. In patients 65 years and older, a higher proportion of patients treated with dapagliflozin had adverse reactions of hypotension [see WARNINGS AND PRECAUTIONS (5.4)].
Saxagliptin
In the seven double-blind, controlled clinical safety and efficacy trials of saxagliptin, a total of 4751 (42.0%) of the 11,301 patients randomized to saxagliptin were 65 years and over, and 1210 (10.7%) were 75 years and over. No overall differences in safety or effectiveness were observed between subjects ≥65 years old and younger subjects. While this clinical experience has not identified differences in responses between the elderly and younger patients, greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
QTERN is contraindicated in patients with moderate to severe renal impairment (eGFR less than 45 mL/min/1.73 m2), ESRD, or on dialysis [see DOSAGE AND ADMINISTRATION (2.3), CONTRAINDICATIONS (4) and WARNINGS AND PRECAUTIONS (5.4)].
Dapagliflozin
Dapagliflozin was evaluated in two glycemic control studies that included patients with moderate renal impairment (an eGFR of 45 to less than 60 mL/min/1.73 m2, and an eGFR of 30 to less than 60 mL/min/1.73 m2). Patients with diabetes and renal impairment using dapagliflozin for glycemic control may be more likely to experience hypotension and may be at higher risk for acute kidney injury secondary to volume depletion. In the study of patients with an eGFR 30 to less than 60 mL/min/1.73 m2, 13 patients receiving dapagliflozin experienced bone fractures compared to none receiving placebo.
8.7 Hepatic Impairment
QTERN may be used in patients with hepatic impairment. However, the benefit-risk for the use of QTERN in patients with severe hepatic impairment should be individually assessed since safety and efficacy have not been studied in this population [see CLINICAL PHARMACOLOGY (12.3)].
-
10 OVERDOSAGE
In the event of an overdose, contact the Poison Control Center. Appropriate supportive treatment should be initiated as dictated by the patient’s clinical status. The removal of dapagliflozin by hemodialysis has not been studied. Saxagliptin and its major metabolite can be removed by hemodialysis (23% of dose over 4 hours).
-
11 DESCRIPTION
QTERN tablets for oral use contain dapagliflozin and saxagliptin.
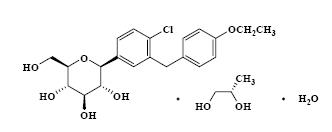
Dapagliflozin propanediol is an active inhibitor of sodium‑glucose cotransporter 2 (SGLT2). It is described chemically as D‑glucitol, 1,5‑anhydro‑1‑C‑[4‑chloro‑3‑[(4‑ethoxyphenyl)methyl]phenyl]‑, (1S)‑. Dapagliflozin is compounded with (2S)‑1,2‑propanediol, hydrate (1:1:1) with an empirical formula as C21H25ClO6•C3H8O2•H2O and the molecular weight of 502.98. The structural formula is:
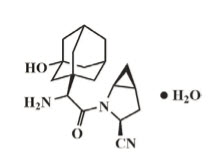
Saxagliptin is an active inhibitor of the dipeptidyl‑peptidase‑4 (DPP‑4) enzyme. It is isolated in the monohydrate form chemically known as (1S,3S,5S)‑2‑[(2S)‑2‑amino‑2‑(3‑hydroxytricyclo [3.3.1.1] dec‑1‑yl)acetyl]‑2‑azabicyclo[3.1.0]hexane‑3‑carbonitrile, monohydrate or (1S,3S,5S)‑2‑[(2S)‑2‑amino‑2‑(3‑hydroxy‑1‑adamantan‑1‑yl)acetyl]‑2‑azabicyclo[3.1.0]hexane‑3‑carbonitrile hydrate. The empirical formula is C18H25N3O2•H2O and the molecular weight is 333.43. The structural formula is:
QTERN is available as film-coated tablets of two strengths:
- •
- 5 mg dapagliflozin/5 mg saxagliptin. Each tablet contains 5 mg dapagliflozin (equivalent to 6.15 mg dapagliflozin propanediol) and 5 mg saxagliptin (exists in the form of HCl salt).
- •
- 10 mg dapagliflozin/5 mg saxagliptin. Each tablet contains 10 mg dapagliflozin (equivalent to 12.3 mg dapagliflozin propanediol) and 5 mg saxagliptin (exists in the form of HCl salt).
Each tablet also contains the following inactive ingredients: anhydrous lactose, croscarmellose sodium, iron oxides, magnesium stearate, microcrystalline cellulose, polyvinyl alcohol, polyethylene glycol, silicon dioxide, talc, and titanium dioxide. Hydrochloric acid and sodium hydroxide (if needed) are added for pH adjustment.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Dapagliflozin
Sodium-glucose cotransporter 2 (SGLT2), expressed in the proximal renal tubules, is responsible for the majority of the reabsorption of filtered glucose from the tubular lumen. Dapagliflozin is an inhibitor of SGLT2. By inhibiting SGLT2, dapagliflozin reduces reabsorption of filtered glucose and thereby promotes urinary glucose excretion.
Saxagliptin
Increased concentrations of the incretin hormones such as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are released into the bloodstream from the small intestine in response to meals. These hormones cause insulin release from the pancreatic beta cells in a glucose-dependent manner but are inactivated by the DPP-4 enzyme within minutes. GLP-1 also lowers glucagon secretion from pancreatic alpha cells, reducing hepatic glucose production. In patients with type 2 diabetes mellitus, concentrations of GLP-1 are reduced but the insulin response to GLP-1 is preserved. Saxagliptin is a competitive DPP-4 inhibitor that slows the inactivation of the incretin hormones, thereby increasing their bloodstream concentrations and reducing fasting and postprandial glucose concentrations in a glucose-dependent manner in patients with type 2 diabetes mellitus.
12.2 Pharmacodynamics
Dapagliflozin
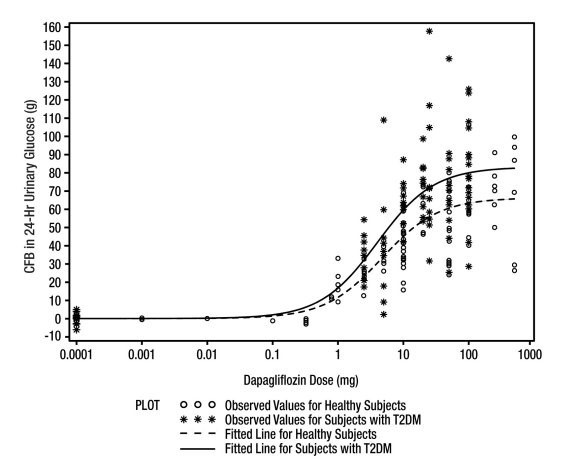
Increases in the amount of glucose excreted in the urine were observed in healthy subjects and in patients with type 2 diabetes mellitus following the administration of dapagliflozin. Dapagliflozin dose of 5 or 10 mg per day in patients with type 2 diabetes mellitus for 12 weeks resulted in excretion of approximately 70 grams of glucose in the urine per day at Week 12. A near maximum glucose excretion was observed at the dapagliflozin daily dose of 20 mg. This urinary glucose excretion with dapagliflozin also results in increases in urinary volume [see ADVERSE REACTIONS (6.1)]. After discontinuation of dapagliflozin, on average, the elevation in urinary glucose excretion approaches baseline by about 3 days from discontinuation for the 10 mg dose.
Figure 1: Scatter Plot and Fitted Line of Change from Baseline in 24-Hour Urinary Glucose Amount versus Dapagliflozin Dose in Healthy Subjects and Subjects with Type 2 Diabetes Mellitus (T2DM) (Semi-Log Plot)
Saxagliptin
In patients with type 2 diabetes mellitus, administration of saxagliptin inhibits DPP-4 enzyme activity for a 24-hour period. After an oral glucose load or a meal, this DPP-4 inhibition resulted in a 2- to 3-fold increase in circulating levels of active GLP-1 and GIP, decreased glucagon concentrations, and increased glucose-dependent insulin secretion from pancreatic beta cells. The rise in insulin and decrease in glucagon were associated with lower fasting glucose concentrations and reduced glucose excursion following an oral glucose load or a meal.
Cardiac Electrophysiology
Dapagliflozin
Dapagliflozin was not associated with clinically meaningful prolongation of QTc interval at daily doses up to 150 mg (15-times the recommended maximum dose) in a study of healthy subjects. In addition, no clinically meaningful effect on QTc interval was observed following single doses of up to 500 mg (50-times the recommended maximum daily dose) of dapagliflozin in healthy subjects.
Saxagliptin
In a randomized, double-blind, placebo-controlled, 4-way crossover, active comparator study using moxifloxacin in 40 healthy subjects, saxagliptin was not associated with clinically meaningful prolongation of the QTc interval or heart rate at daily doses up to 40 mg (8-times the recommended maximum daily dose).
12.3 Pharmacokinetics
Overall, the pharmacokinetics of dapagliflozin and saxagliptin were not affected in a clinically relevant manner when administered as QTERN.
Saxagliptin
The pharmacokinetics of saxagliptin and its active metabolite, 5-hydroxy saxagliptin, were similar in healthy subjects and in patients with type 2 diabetes mellitus. The Cmax and AUC values of saxagliptin and its active metabolite increased proportionally in the 2.5 to 400 mg dose range. Following a 5 mg single oral dose of saxagliptin to healthy subjects, the mean plasma AUC values for saxagliptin and its active metabolite were 78 ng•h/mL and 214 ng•h/mL, respectively. The corresponding plasma Cmax values were 24 ng/mL and 47 ng/mL, respectively. The average variability (%CV) for AUC and Cmax for both saxagliptin and its active metabolite was less than 25%.
No appreciable accumulation of either saxagliptin or its active metabolite was observed with repeated once daily dosing at any dose level. No dose- and time-dependence were observed in the clearance of saxagliptin and its active metabolite over 14 days of once daily dosing with saxagliptin at doses ranging from 2.5 to 400 mg.
Absorption
Dapagliflozin
Following oral administration of dapagliflozin, the maximum plasma concentration (Cmax) is usually attained within 2 hours under fasting state. The Cmax and AUC values increase dose proportionally with increase in dapagliflozin dose in the therapeutic dose range. The absolute oral bioavailability of dapagliflozin following the administration of a 10 mg dose is 78%. Administration of dapagliflozin with a high-fat meal decreases its Cmax by up to 50% and prolongs Tmax by approximately 1 hour but does not alter AUC as compared with the fasted state.
Saxagliptin
The median time to maximum concentration (Tmax) following the 5 mg once daily dose was 2 hours for saxagliptin and 4 hours for its active metabolite. Administration with a high-fat meal resulted in an increase in Tmax of saxagliptin by approximately 20 minutes as compared to fasted conditions. There was a 27% increase in the AUC of saxagliptin when given with a meal as compared to fasted conditions.
Distribution
Dapagliflozin
Dapagliflozin is approximately 91% protein bound. Protein binding is not altered in patients with renal or hepatic impairment.
Saxagliptin
The in vitro protein binding of saxagliptin and its active metabolite in human serum is negligible. Therefore, changes in blood protein levels in various disease states (e.g., renal or hepatic impairment) are not expected to alter the disposition of saxagliptin.
Metabolism
Dapagliflozin
The metabolism of dapagliflozin is primarily mediated by UGT1A9; CYP-mediated metabolism is a minor clearance pathway in humans. Dapagliflozin is extensively metabolized, primarily to yield dapagliflozin 3-O-glucuronide, which is an inactive metabolite. Dapagliflozin 3-O-glucuronide accounted for 61% of a 50 mg [14C]-dapagliflozin dose and is the predominant drug-related component in human plasma.
Saxagliptin
The metabolism of saxagliptin is primarily mediated by cytochrome P450 3A4/5 (CYP3A4/5). The major metabolite of saxagliptin is also a DPP-4 inhibitor, which is one-half as potent as saxagliptin. Therefore, strong CYP3A4/5 inhibitors and inducers will alter the pharmacokinetics of saxagliptin and its active metabolite [see DRUG INTERACTIONS (7)].
Elimination
Dapagliflozin
Dapagliflozin and related metabolites are primarily eliminated via the renal pathway. Following a single 50 mg dose of [14C]-dapagliflozin, 75% and 21% total radioactivity is excreted in urine and feces, respectively. In urine, less than 2% of the dose is excreted as parent drug. In feces, approximately 15% of the dose is excreted as parent drug. The mean plasma terminal half-life (t1/2) for dapagliflozin is approximately 12.9 hours following a single oral dose of dapagliflozin 10 mg.
Saxagliptin
Saxagliptin is eliminated by both renal and hepatic pathways. Following a single 50 mg dose of [14C]-saxagliptin, 24%, 36%, and 75% of the dose was excreted in the urine as saxagliptin, its active metabolite, and total radioactivity, respectively. The average renal clearance of saxagliptin (~230 mL/min) was greater than the average estimated glomerular filtration rate (~120 mL/min), suggesting some active renal excretion. A total of 22% of the administered radioactivity was recovered in feces representing the fraction of the saxagliptin dose excreted in bile and/or unabsorbed drug from the gastrointestinal tract. Following a single oral dose of saxagliptin 5 mg to healthy subjects, the mean plasma terminal half-life (t1/2) for saxagliptin and its active metabolite was 2.5 and 3.1 hours, respectively.
Specific Populations
Effects of Age, Gender, Race and Body Weight on Pharmacokinetics
Based on a population pharmacokinetic analysis, age, gender, race, and body weight do not have a clinically meaningful effect on the pharmacokinetics of saxagliptin and dapagliflozin.
Renal Impairment
Dapagliflozin
At steady state (20 mg once daily dapagliflozin for 7 days), patients with type 2 diabetes mellitus with mild, moderate, or severe renal impairment (as determined by eGFR) had geometric mean systemic exposures of dapagliflozin that were 45%, 100%, and 200% higher, respectively, as compared to patients with type 2 diabetes mellitus with normal renal function. Higher systemic exposure of dapagliflozin in patients with type 2 diabetes mellitus with renal impairment did not result in a correspondingly higher 24-hour urinary glucose excretion. The steady-state 24-hour urinary glucose excretion in patients with type 2 diabetes mellitus and mild, moderate, and severe renal impairment was 42%, 80%, and 90% lower, respectively, than in patients with type 2 diabetes mellitus with normal renal function. The impact of hemodialysis on dapagliflozin exposure is not known [see DOSAGE AND ADMINISTRATION (2.3), WARNINGS AND PRECAUTIONS (5.4) and USE IN SPECIFIC POPULATIONS (8.6)].
Saxagliptin
A single-dose, open-label study was conducted to evaluate the pharmacokinetics of saxagliptin (10 mg dose) in subjects with varying degrees of chronic renal impairment compared to subjects with normal renal function. The 10 mg dosage is not an approved dosage. The degree of renal impairment did not affect Cmax of saxagliptin or its metabolite. In subjects with moderate renal impairment (eGFR 30 to less than 45 mL/min/1.73 m2), severe renal impairment (eGFR 15 to less than 30 mL/min/1.73 m2) and ESRD patient on hemodialysis, the AUC values of saxagliptin or its active metabolite were >2-fold higher than AUC values in subjects with normal renal function. QTERN is contraindicated in patients with an eGFR <45 mL/min/1.73 m2.
Hepatic Impairment
Dapagliflozin
In subjects with mild and moderate hepatic impairment (Child-Pugh classes A and B), mean Cmax and AUC of dapagliflozin were up to 12% and 36% higher, respectively, as compared to healthy matched control subjects following single-dose administration of 10 mg dapagliflozin. These differences were not considered to be clinically meaningful. In patients with severe hepatic impairment (Child-Pugh class C), mean Cmax and AUC of dapagliflozin were up to 40% and 67% higher, respectively, as compared to healthy matched controls [see USE IN SPECIFIC POPULATIONS (8.7)].
Saxagliptin
In subjects with hepatic impairment (Child-Pugh classes A, B, and C), mean Cmax and AUC of saxagliptin were up to 8% and 77% higher, respectively, compared to healthy matched controls following administration of a single 10 mg dose of saxagliptin. The 10 mg dosage is not an approved dosage. The corresponding Cmax and AUC of the active metabolite were up to 59% and 33% lower, respectively, compared to healthy matched controls. These differences are not considered to be clinically meaningful.
Pediatric
Pharmacokinetics of QTERN in the pediatric population has not been studied.
Drug Interactions
Saxagliptin and Dapagliflozin
The lack of pharmacokinetic interaction between dapagliflozin and saxagliptin was demonstrated in a drug-drug interaction study between dapagliflozin and saxagliptin.
Dapagliflozin
In Vitro Assessment of Drug Interactions
The metabolism of dapagliflozin is primarily via glucuronide conjugation mediated by UDP glucuronosyltransferase 1A9 (UGT1A9).
In in vitro studies, dapagliflozin and dapagliflozin 3-O-glucuronide neither inhibited CYP 1A2, 2C9, 2C19, 2D6, or 3A4, nor induced CYP 1A2, 2B6, or 3A4. Dapagliflozin is a weak substrate of the P-glycoprotein (P‑gp) active transporter, and dapagliflozin 3-O-glucuronide is a substrate for the OAT3 active transporter. Dapagliflozin or dapagliflozin 3-O-glucuronide did not meaningfully inhibit P‑gp, OCT2, OAT1, or OAT3 active transporters. Overall, dapagliflozin is unlikely to affect the pharmacokinetics of concurrently administered medications that are P‑gp, OCT2, OAT1, or OAT3 substrates.
Effects of Other Drugs on Dapagliflozin
Table 4 shows the effect of coadministered drugs on the pharmacokinetics of dapagliflozin.
Table 4: Effects of Coadministered Drugs on Dapagliflozin Systemic Exposure Coadministered Drug
(Dose Regimen)*Dapagliflozin
(Dose Regimen)*Dapagliflozin
Change† in Cmax
Oral Antidiabetic Agents
Metformin (1000 mg)
20 mg
↓1%
↓7%
Pioglitazone (45 mg)
50 mg
0%
↑9%
Sitagliptin (100 mg)
20 mg
↑8%
↓4%
Glimepiride (4 mg)
20 mg
↓1%
↑1%
Voglibose (0.2 mg three times daily)
10 mg
↑1%
↑4%
Saxagliptin (5 mg single dose)
10 mg (single dose)
↓2%
↓6%
Other Medications
Hydrochlorothiazide (25 mg)
50 mg
↑7%
↓1%
Bumetanide (1 mg)
10 mg once daily for 7 days
↑5%
↑8%
Valsartan (320 mg)
20 mg
↑2%
↓12%
Simvastatin (40 mg)
20 mg
↓1%
↓2%
Anti‑infective Agent
Rifampin (600 mg once daily for 6 days)
10 mg
↓22%
↓7%
Nonsteroidal Anti‑inflammatory Agent
Mefenamic Acid (loading dose of 500 mg followed by 14 doses
of 250 mg every 6 hours)10 mg
↑51%
↑13%
Effects of Dapagliflozin on Other Drugs
Table 5 shows the effect of dapagliflozin on other coadministered drugs. Dapagliflozin did not meaningfully affect the pharmacokinetics of the coadministered drugs.
Table 5: Effects of Dapagliflozin on the Systemic Exposures of Coadministered Drugs Coadministered Drug
(Dose Regimen)*Dapagliflozin
(Dose Regimen)*Coadministered Drug Change† in AUC‡ Change† in Cmax Oral Antidiabetic Agents
Metformin (1000 mg)
20 mg
0%
↓5%
Pioglitazone (45 mg)
50 mg
0%
↓7%
Sitagliptin (100 mg)
20 mg
↑1%
↓11%
Glimepiride (4 mg)
20 mg
↑13%
↑4%
Other Medications
Hydrochlorothiazide (25 mg)
50 mg
↓1%
↓5%
Bumetanide (1 mg)
10 mg once daily
for 7 days↑13%
↑13%
Valsartan (320 mg)
20 mg
↑5%
↓6%
Simvastatin (40 mg)
20 mg
↑19%
↓6%
Digoxin (0.25 mg)
20 mg loading dose then 10 mg once daily for 7 days
0%
↓1%
Warfarin (25 mg)
S‑warfarin
R‑warfarin20 mg loading dose then 10 mg once daily for 7 days
↑3%
↑6%↑7%
↑8%Saxagliptin
In Vitro Assessment of Drug Interactions
The metabolism of saxagliptin is primarily mediated by CYP3A4/5.
In in vitro studies, saxagliptin and its active metabolite did not inhibit CYP1A2, 2A6, 2B6, 2C9, 2C19, 2D6, 2E1, or 3A4, or induce CYP1A2, 2B6, 2C9, or 3A4. Therefore, saxagliptin is not expected to alter the metabolic clearance of coadministered drugs that are metabolized by these enzymes. Saxagliptin is a P-glycoprotein (P‑gp) substrate but is not a significant inhibitor or inducer of P‑gp.
Effects of Other Drugs on Saxagliptin and its Active Metabolite, 5-hydroxy Saxagliptin
Table 6: Effect of Coadministered Drugs on Systemic Exposures of Saxagliptin and its Active Metabolite, 5-hydroxy Saxagliptin Coadministered Drug Dosage of
Coadministered Drug*Dosage of Saxagliptin* Saxagliptin Change† in AUC‡ Change† in Cmax - *
- Single dose unless otherwise noted.
- †
- Percent change (with/without coadministered drug and no change=0%); ↑ and ↓ indicate the exposure increase and decrease, respectively.
- ‡
- AUC=AUC(INF) for drugs given as single dose and AUC=AUC(TAU) for drugs given in multiple doses.
- §
- Results exclude one subject.
- ¶
- The plasma dipeptidyl peptidase-4 (DPP-4) activity inhibition over a 24-hour dose interval was not affected by rifampin.
Metformin
1000 mg
100 mg
saxagliptin
5‑hydroxy saxagliptin↓2%
↓1%↓21%
↓12%Glyburide
5 mg
10 mg
saxagliptin
5‑hydroxy saxagliptin↓2%
ND↑8%
NDPioglitazone§
45 mg QD for 10 days
10 mg QD
for 5 dayssaxagliptin
5‑hydroxy saxagliptin↑11%
ND↑11%
NDDapagliflozin
10 mg single dose
5 mg single dose
saxagliptin
5-hydroxy saxagliptin
↓1%
↑9%
↓7%
↑6%
Digoxin
0.25 mg q6h first day followed by q12h second day followed by QD for 5 days
10 mg QD
for 7 dayssaxagliptin
5‑hydroxy saxagliptin↑5%
↑6%↓1%
↑2%Simvastatin
40 mg QD for 8 days
10 mg QD
for 4 dayssaxagliptin
5‑hydroxy saxagliptin↑12%
↑2%↑21%
↑8%Diltiazem
360 mg LA QD for 9 days
10 mg
saxagliptin
5‑hydroxy saxagliptin↑109%
↓34%↑63%
↓43%Rifampin¶
600 mg QD for 6 days
5 mg
saxagliptin
5‑hydroxy saxagliptin↓76%
↑3%↓53%
↑39%Omeprazole
40 mg QD for 5 days
10 mg
saxagliptin
5‑hydroxy saxagliptin↑13%
ND↓2%
NDAluminum hydroxide + magnesium hydroxide + simethicone
aluminum hydroxide:
2400 mg
magnesium hydroxide: 2400 mg simethicone:
240 mg10 mg
saxagliptin
5‑hydroxy saxagliptin↓3%
ND↓26%
NDFamotidine
40 mg
10 mg
saxagliptin
5‑hydroxy saxagliptin↑3%
ND↑14%
NDSaxagliptin coadministered with strong CYP3A4/5 inhibitors [see DRUG INTERACTIONS (7) and DOSAGE AND ADMINISTRATION (2.4)]:
Ketoconazole
200 mg BID for 9 days
100 mg
saxagliptin
5‑hydroxy saxagliptin↑145%
↓88%↑62%
↓95%Ketoconazole
200 mg BID for 7 days
20 mg
saxagliptin
5‑hydroxy saxagliptin↑267%
ND↑144%
NDND=not determined; QD=once daily; q6h=every 6 hours; q12h=every 12 hours; BID=twice daily; LA=long acting.
Effects of Saxagliptin on Other Drugs
Table 7: Effect of Saxagliptin on Systemic Exposures of Coadministered Drugs Coadministered Drug Dosage of
Coadministered Drug*Dosage of
Saxagliptin*Coadministered Drug Change† in AUC‡ Change†
in Cmax- *
- Single dose unless otherwise noted.
- †
- Percent change (with/without coadministered drug and no change=0%); ↑ and ↓ indicate the exposure increase and decrease, respectively.
- ‡
- AUC=AUC(INF) for drugs given as single dose and AUC=AUC(TAU) for drugs given in multiple doses.
- §
- Results include all subjects.
Metformin
1000 mg
100 mg
metformin
↑20%
↑9%
Glyburide
5 mg
10 mg
glyburide
↑6%
↑16%
Pioglitazone§
45 mg QD for 10 days
10 mg QD
for 5 dayspioglitazone
hydroxy‑pioglitazone↑8%
ND↑14%
NDDigoxin
0.25 mg q6h first day followed by q12h second day followed by QD for 5 days
10 mg QD
for 7 daysdigoxin
↑6%
↑9%
Simvastatin
40 mg QD for 8 days
10 mg QD
for 4 dayssimvastatin
simvastatin acid↑4%
↑16%↓12%
0%Diltiazem
360 mg LA QD for 9 days
10 mg
diltiazem
↑10%
↑16%
Ketoconazole
200 mg BID for 9 days
100 mg
ketoconazole
↓13%
↓16%
Ethinyl estradiol and Norgestimate
ethinyl estradiol 0.035 mg and norgestimate 0.250 mg for 21 days
5 mg QD for 21 days
ethinyl estradiol
norelgestromin
norgestrel↑7%
↑10%
↑13%↓2%
↑9%
↑17%- ND=not determined; QD=once daily; q6h=every 6 hours; q12h=every 12 hours; BID=twice daily; LA=long acting.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
QTERN
No animal studies have been conducted with the combined products in QTERN to evaluate carcinogenesis, mutagenesis, or impairment of fertility. The following data are based on the findings in the studies with dapagliflozin and saxagliptin individually.
Dapagliflozin
Carcinogenesis
Dapagliflozin did not induce tumors in either mice or rats at any of the doses evaluated in 2-year carcinogenicity studies. Oral doses in mice consisted of 5, 15, and 40 mg/kg/day in males and 2, 10, and 20 mg/kg/day in females, and oral doses in rats were 0.5, 2, and 10 mg/kg/day for both males and females. The highest doses evaluated in mice were approximately 72-times (males) and 105-times (females) the clinical dose of 10 mg per day, based on AUC exposure. In rats, the highest dose was approximately 131-times (males) and 186-times (females) the clinical dose of 10 mg per day, based on AUC exposure.
Mutagenesis
Dapagliflozin was negative in the Ames mutagenicity assay and was positive in a series of in vitro clastogenicity assays in the presence of S9 activation and at concentrations greater than or equal to 100 µg/mL. Dapagliflozin was negative for clastogenicity in a series of in vivo studies evaluating micronuclei or DNA repair in rats at exposure multiples greater than 2100-times the clinical dose.
Impairment of Fertility
Dapagliflozin had no effects on mating, fertility, or early embryonic development in treated male or female rats at exposure multiples less than or equal to 1708-times and 998-times the maximum recommended human dose in males and females, respectively.
Saxagliptin
Carcinogenesis
Carcinogenicity was evaluated in 2-year studies conducted in CD-1 mice and Sprague-Dawley rats. Saxagliptin did not increase the incidence of tumors in mice dosed orally at 50, 250, and 600 mg/kg up to 870-times (males) and 1165-times (females) the 5 mg/day clinical dose, based on AUC. Saxagliptin did not increase the incidence of tumors in rats dosed orally at 25, 75, 150, and 300 mg/kg up to 355-times (males) and 2217-times (females) the 5 mg/day clinical dose, based on AUC.
Mutagenesis
Saxagliptin was not mutagenic or clastogenic in a battery of genotoxicity tests (Ames bacterial mutagenesis, human and rat lymphocyte cytogenetics, rat bone marrow micronucleus and DNA repair assays). The active metabolite of saxagliptin was not mutagenic in an Ames bacterial assay.
Impairment of Fertility
Saxagliptin administered to rats had no effect on fertility or the ability to maintain a litter at exposures up to 603-times and 776-times the 5 mg clinical dose in males and females, based on AUC.
13.2 Animal Toxicology and/or Pharmacology
Saxagliptin
Saxagliptin produced adverse skin changes in the extremities of cynomolgus monkeys (scabs and/or ulceration of tail, digits, scrotum, and/or nose). Skin lesions were reversible within exposure approximately 20-times the 5 mg clinical dose, but in some cases were irreversible and necrotizing at higher exposures. Adverse skin changes were not observed at exposures similar to (1- to 3-times) the 5 mg clinical dose. Clinical correlates to skin lesions in monkeys have not been observed in human clinical trials of saxagliptin.
-
14 CLINICAL STUDIES
The dapagliflozin and saxagliptin in combination with metformin has been studied in adult patients with type 2 diabetes mellitus (T2DM) inadequately controlled on metformin in the following studies.
Treatment with dapagliflozin and saxagliptin and metformin (combination or add-on therapy) at all doses produced statistically significant improvements in HbA1c compared to the active comparator or placebo study arms in combination with metformin.
14.1 Add-on Therapy with Dapagliflozin plus Saxagliptin in Patients on Metformin
Adult patients with inadequately controlled type 2 diabetes mellitus participated in 2 active-controlled studies of 24-week duration to evaluate therapy with 5 mg dapagliflozin/5 mg saxagliptin or 10 mg dapagliflozin/5 mg saxagliptin combinations on a background of metformin.
One study was a 24-week randomized, double-blind, active-controlled, parallel-group study (NCT02681094) in T2DM patients with an HbA1c ≥7.5% and ≤10.0%. Patients were on a stable dose of metformin HCl (≥1500 mg per day) for at least 8 weeks prior to being randomized to one of three double-blind treatment groups to receive 5 mg dapagliflozin and 5 mg saxagliptin added to metformin, 5 mg saxagliptin and placebo added to metformin, or 5 mg dapagliflozin and placebo added to metformin.
At Week 24, concomitant addition of 5 mg dapagliflozin and 5 mg saxagliptin plus metformin resulted in statistically significant decreases in HbA1c, and a larger proportion of patients achieving the therapeutic glycemic goal of HbA1c <7%, compared to dapagliflozin plus metformin or saxagliptin plus metformin (see Table 8).
Table 8: HbA1c Results at Week 24 with the Combination of 5 mg Dapagliflozin and 5 mg Saxagliptin plus Metformin* Efficacy Parameter 5 mg Dapagliflozin and 5 mg Saxagliptin + Metformin 5 mg Dapagliflozin and 5 mg Saxagliptin +
Metformin5 mg Dapagliflozin +
Metformin5 mg Saxagliptin +
Metformin- *
- Analysis of Covariance including all post-baseline data regardless of rescue or treatment discontinuation. Model estimates calculated using multiple imputation to model washout of the treatment effect using control arm data for all subjects having missing Week 24 data.
- †
- The number of randomized subjects who took at least one dose of double-blind study medication and had a baseline value for HbA1c.
- ‡
- p-value <0.0001.
- §
- p-value <0.0001 vs. dapagliflozin and saxagliptin plus metformin.
- ¶
- p-value = 0.0018 vs. dapagliflozin and saxagliptin plus metformin.
N†
290
289
291
Baseline (mean)
8.1
8.2
8.3
Change from baseline (adjusted mean)
(95% CI)‑1.02
(‑1.13, ‑0.90)
‑0.62
(‑0.73, ‑0.51)
‑0.69
(‑0.80, ‑0.59)
Difference from dapagliflozin + metformin
(adjusted mean)
(95% CI)‑0.40‡
(‑0.55, ‑0.24)
Difference from saxagliptin + metformin (adjusted mean)
(95% CI)‑0.32‡
(‑0.48, ‑0.17)
Percent of patients achieving HbA1c <7%
42.8
21.8§
28.5¶
The adjusted mean change from baseline for body weight at Week 24, using values regardless of rescue or treatment discontinuation, was -2.0 kg for the 5 mg dapagliflozin and 5 mg saxagliptin plus metformin group, -2.1 kg for the 5 mg dapagliflozin plus metformin group, and -0.4 kg for the 5 mg saxagliptin plus metformin group. The difference in mean body weight between the 5 mg dapagliflozin and 5 mg saxagliptin plus metformin group and the 5 mg dapagliflozin plus metformin group was -1.6 kg (95% CI [-2.1, -1.0]).
The second study was a 24-week randomized, double-blind, active comparator-controlled superiority study (NCT016060007) that compared once daily 10 mg dapagliflozin and 5 mg saxagliptin coadministered in combination with metformin XR with either 10 mg dapagliflozin and placebo added to metformin or 5 mg saxagliptin and placebo added to metformin in T2DM adult patients with inadequate glycemic control on metformin alone (HbA1c ≥8% and ≤12%).
At Week 24, concomitant addition of 10 mg dapagliflozin and 5 mg saxagliptin plus metformin resulted in statistically significant decreases in HbA1c, and a larger proportion of patients achieving an HbA1c <7%, compared to dapagliflozin plus metformin or saxagliptin plus metformin (see Table 9).
Table 9: HbA1c Results at Week 24 with the Combination of 10 mg Dapagliflozin and 5 mg Saxagliptin plus Metformin* Efficacy Parameter 10 mg Dapagliflozin and
5 mg Saxagliptin + Metformin10 mg Dapagliflozin and 5 mg Saxagliptin +
Metformin10 mg Dapagliflozin +
Metformin5 mg Saxagliptin
+
Metformin- *
- Analysis of Covariance including all post-baseline data regardless of rescue or treatment discontinuation. Model estimates calculated using multiple imputation to model washout of the treatment effect using control arm data for all subjects having missing Week 24 data.
- †
- The number of randomized subjects who took at least one dose of double-blind study medication and had a baseline value for HbA1c.
- ‡
- p-value=0.0148.
- §
- p-value <0.0001.
- ¶
- Not statistically significant based on the prespecified method for controlling type I error.
N†
179
179
176
Baseline (mean)
8.9
8.9
9.0
Change from baseline (adjusted mean)
(95% CI)−1.49
(−1.64, −1.34)
−1.23
(−1.38, −1.08)
−1.00
(−1.15, −0.85)
Difference from dapagliflozin + metformin
(adjusted mean)
(95% CI)−0.26‡
(−0.47, −0.05)
Difference from saxagliptin + metformin (adjusted mean)
(95% CI)−0.49§
(−0.70, −0.27)
Percent of patients achieving HbA1c <7%
40.2¶
21.2¶
16.5¶
The adjusted mean change from baseline for body weight at Week 24, using values regardless of rescue or treatment discontinuation, was -2.0 kg for the 10 mg dapagliflozin and 5 mg saxagliptin plus metformin group, -2.3 kg for the 10 mg dapagliflozin plus metformin group, and 0 kg for the 5 mg saxagliptin plus metformin group.
14.2 Add-on Therapy with Saxagliptin in Patients on Dapagliflozin plus Metformin
A total of 315 patients with type 2 diabetes mellitus participated in this 24-week randomized, double-blind, placebo-controlled trial to evaluate the efficacy and safety of saxagliptin added to dapagliflozin and metformin in patients with a baseline of HbA1c ≥7% to ≤10.5% (NCT01619059). The mean age of these subjects was 54.6 years, 1.6% were 75 years or older and 52.7% were female. The population was 87.9% White, 6.3% Black or African American, 4.1% Asian, and 1.6% Other race. At baseline the population had diabetes for an average of 7.7 years and a mean HbA1c of 7.9%. The mean eGFR at baseline was 93.4 mL/min/1.73 m2. Patients were required to be on a stable dose of metformin (≥1500 mg per day) for at least 8 weeks prior to enrollment. Eligible subjects who completed the screening period entered the lead-in treatment period, which included 16 weeks of open-label metformin and 10 mg dapagliflozin treatment. Following the lead-in period, eligible patients were randomized to 5 mg saxagliptin (N=153) or placebo (N=162).
The group treated with add-on saxagliptin had statistically significant greater reductions in HbA1c from baseline versus the group treated with placebo (see Table 10).
Table 10: HbA1c Change from Baseline at Week 24 in a Placebo-Controlled Trial of Saxagliptin as Add-on to Dapagliflozin and Metformin* - *
- There were 6.5% (n=10) of randomized subjects in the saxagliptin arm and 3.1% (n=5) in the placebo arm for whom change from baseline HbA1c data was missing at Week 24. Of the subjects who discontinued study medication early, 9.1% (1 of 11) in the saxagliptin arm and 16.7% (1 of 6) in the placebo arm had HbA1c measured at Week 24.
- †
- N is the number of randomized and treated patients.
- ‡
- Analysis of Covariance including all post-baseline data regardless of rescue or treatment discontinuation. Model estimates calculated using multiple imputation to model washout of the treatment effect using placebo data for all subjects having missing Week 24 data.
- §
- Least squares mean adjusted for baseline value.
- ¶
- p-value <0.0001.
Efficacy parameter
5 mg Saxagliptin
(N=153)†
Placebo
(N=162)†
In combination with Dapagliflozin and Metformin
HbA1c (%) at week 24‡
Baseline (mean)
8.0
7.9
Change from baseline (adjusted mean§)
95% Confidence Interval
−0.5
(−0.6, −0.4)
−0.2
(−0.3, −0.1)
Difference from placebo (adjusted mean)
95% Confidence Interval
−0.4¶
(−0.5, −0.2)
Percent of patients achieving HbA1c <7%
35.3
23.1
14.3 Cardiovascular Safety Trial
The cardiovascular risk of saxagliptin was evaluated in SAVOR (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus - Thrombolysis in Myocardial Infarction), a multicenter, multinational, randomized, double-blind trial comparing saxagliptin (N=8280) to placebo (N=8212), in adult patients with type 2 diabetes mellitus at high risk for atherosclerotic cardiovascular disease. Of the randomized study subjects, 97.5% completed the trial, and the median duration of follow-up was approximately 2 years (NCT01107886).
Subjects were at least 40 years of age, had HbA1c ≥6.5%, and multiple risk factors (21% of randomized subjects) for cardiovascular disease (age ≥55 years for men and ≥60 years for women plus at least one additional risk factor of dyslipidemia, hypertension, or current cigarette smoking) or established (79% of the randomized subjects) cardiovascular disease defined as a history of ischemic heart disease, peripheral vascular disease, or ischemic stroke. Overall, the use of diabetes medications was balanced across treatment groups (metformin 69%, insulin 41%, sulfonylureas 40%, and TZDs 6%). The use of cardiovascular disease medications was also balanced (angiotensin-converting enzyme [ACE] inhibitors or angiotensin receptor blockers [ARBs] 79%, statins 78%, aspirin 75%, beta-blockers 62%, and non-aspirin antiplatelet medications 24%).
The majority of subjects were male (67%) and Caucasian (75%) with a mean age of 65 years. Approximately 16% of the population had moderate (eGFR ≥30 to ≤50 mL/min/1.73 m2) to severe (eGFR <30 mL/min/1.73 m2) renal impairment, and 13% had a prior history of heart failure. QTERN is contraindicated in patients with an eGFR <45 mL/min/1.73 m2. Subjects had a median duration of type 2 diabetes mellitus of approximately 10 years and a mean baseline HbA1c level of 8.0%.
The primary analysis in SAVOR was time to first occurrence of a Major Adverse Cardiac Event (MACE). A major adverse cardiac event in SAVOR was defined as a cardiovascular death or a nonfatal myocardial infarction (MI) or a nonfatal ischemic stroke. The incidence rate of MACE was similar in both treatment arms: 3.8 MACE per 100 patient-years on placebo vs. 3.8 MACE per 100 patient-years on saxagliptin with an estimated HR: 1.0; 95.1% CI: (0.89, 1.12). The upper bound of this confidence interval, 1.12, excluded a risk margin larger than 1.3.
Vital status was obtained for 99% of subjects in the trial. There were 798 deaths in the SAVOR trial. Numerically more patients (5.1%) died in the saxagliptin group than in the placebo group (4.6%). The risk of deaths from all-cause mortality was not statistically different between the treatment groups (HR: 1.11; 95.1% CI: 0.96, 1.27).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
QTERN® (dapagliflozin and saxagliptin) tablets are available in packages as listed:
Table 11: QTERN Tablet Presentations Tablet Strength
Film‑Coated Tablet Color / Shape
Tablet Markings
Pack Size
NDC Code
5 mg dapagliflozin /5 mg saxagliptin
Light purple to reddish purple, biconvex, round
“1120” printed on both sides, in blue ink
Bottles of 30
Bottles of 90
Bottles of 500
0310-6770-30
0310-6770-90
0310-6770-50
10 mg dapagliflozin /5 mg saxagliptin
Light brown to brown, biconvex, round
“1122” printed on both sides, in blue ink
Bottles of 30
Bottles of 90
Bottles of 500
0310‑6780‑30
0310‑6780‑90
0310‑6780‑50
Storage and Handling
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Diabetic Ketoacidosis in Patients with Type 1 Diabetes Mellitus and Other Ketoacidosis
- •
- Inform patients that QTERN can cause potentially fatal ketoacidosis and that type 2 diabetes mellitus and pancreatic disorders (e.g., history of pancreatitis or pancreatic surgery) are risk factors.
- •
- Educate all patients on precipitating factors (such as insulin dose reduction or missed insulin doses, infection, reduced caloric intake, ketogenic diet, surgery, dehydration, and alcohol abuse) and symptoms of ketoacidosis (including nausea, vomiting, abdominal pain, tiredness, and labored breathing). Inform patients that blood glucose may be normal even in the presence of ketoacidosis.
- •
- Advise patients that they may be asked to monitor ketones. If symptoms of ketoacidosis occur, instruct patients to discontinue QTERN and seek medical attention immediately [see WARNINGS AND PRECAUTIONS (5.1)].
Pancreatitis
- •
- Inform patients that acute pancreatitis has been reported during postmarketing use of saxagliptin. Inform patients that persistent severe abdominal pain, sometimes radiating to the back, which may or may not be accompanied by vomiting, is the hallmark symptom of acute pancreatitis.
- •
- Instruct patients to promptly discontinue QTERN and contact their healthcare provider if persistent severe abdominal pain occurs [see WARNINGS AND PRECAUTIONS (5.2)].
Heart Failure
- •
- Inform patients of the signs and symptoms of heart failure. Instruct patients to contact their healthcare provider as soon as possible if they experience symptoms of heart failure, including increasing shortness of breath, rapid increase in weight or swelling of the feet [see WARNINGS AND PRECAUTIONS (5.3)].
Volume Depletion
- •
- Inform patients that symptomatic hypotension may occur with QTERN and advise them to contact their healthcare provider if they experience such symptoms. Inform patients that dehydration may increase the risk for hypotension, and to have adequate fluid intake [see WARNINGS AND PRECAUTIONS (5.4)].
Serious Urinary Tract Infections
- •
- Inform patients of the potential for urinary tract infections, which may be serious. Inform them of the symptoms of urinary tract infections and advise them to seek medical advice if such symptoms occur [see WARNINGS AND PRECAUTIONS (5.5)].
Hypoglycemia with Concomitant Use of Insulin or Insulin Secretagogues
- •
- Inform patients that the incidence of hypoglycemia may increase when QTERN is added to an insulin secretagogue (e.g., sulfonylurea) and/or insulin. Educate patients on the signs and symptoms of hypoglycemia [see WARNINGS AND PRECAUTIONS (5.6)].
Necrotizing Fasciitis of the Perineum (Fournier’s Gangrene)
- •
- Inform patients that necrotizing infections of the perineum (Fournier’s gangrene) have occurred with dapagliflozin, a component of QTERN. Counsel patients to promptly seek medical attention if they develop pain or tenderness, redness, or swelling of the genitals or the area from the genitals back to the rectum, along with a fever above 100.4°F or malaise [see WARNINGS AND PRECAUTIONS (5.7)].
Hypersensitivity Reactions
- •
- Inform patients that serious hypersensitivity reactions (e.g., anaphylaxis, angioedema, urticaria, and exfoliative skin conditions) have been reported with dapagliflozin and saxagliptin, components of QTERN. Symptoms of these allergic reactions include: rash, skin flaking or peeling, urticaria, swelling of the skin, or swelling of the face, lips, tongue, and throat that may cause difficulty in breathing or swallowing.
- •
- Advise patients to immediately report any signs or symptoms suggesting allergic reaction, angioedema or exfoliative skin conditions, and stop taking QTERN and seek medical advice promptly [see WARNINGS AND PRECAUTIONS (5.8)].
Genital Mycotic Infections in Females (e.g., Vulvovaginitis)
- •
- Inform female patients that vaginal yeast infections may occur and provide them with information on the signs and symptoms of vaginal yeast infections. Advise them of treatment options and when to seek medical advice [see WARNINGS AND PRECAUTIONS (5.9)].
Genital Mycotic Infections in Males (e.g., Balanitis or Balanoposthitis)
- •
- Inform male patients that yeast infections of the penis (e.g., balanitis or balanoposthitis) may occur, especially in patients with prior history. Provide them with information on the signs and symptoms of balanitis and balanoposthitis (rash or redness of the glans or foreskin of the penis). Advise them of treatment options and when to seek medical advice [see WARNINGS AND PRECAUTIONS (5.9)].
Severe and Disabling Arthralgia
- •
- Inform patients that severe and disabling joint pain may occur with this class of drugs. The time to onset of symptoms can range from one day to years. Instruct patients to seek medical advice if severe joint pain occurs [see WARNINGS AND PRECAUTIONS (5.10)].
Bullous Pemphigoid
- •
- Inform patients that bullous pemphigoid may occur with QTERN. Instruct patients to seek medical advice if blisters or erosions occur [see WARNINGS AND PRECAUTIONS (5.11)].
Pregnancy
- •
- Advise pregnant patients of the potential risk to a fetus with treatment with QTERN. Instruct patients to immediately inform their healthcare provider if pregnant or planning to become pregnant [see USE IN SPECIFIC POPULATIONS (8.1)].
Lactating Mothers
- •
- Advise patients that use of QTERN is not recommended while breastfeeding [see USE IN SPECIFIC POPULATIONS (8.2)].
Laboratory Tests
- •
- Inform patients that due to its mechanism of action, patients taking QTERN will test positive for glucose in their urine.
Administration
- •
- Instruct patients that QTERN must be swallowed whole and not crushed or chewed.
Missed Dose
- •
- If a dose is missed, advise patients to take it as soon as it is remembered unless it is almost time for the next dose, in which case patients should skip the missed dose and take the medicine at the next regularly scheduled time. Advise patients not to take two doses of QTERN at the same time.
Distributed by:
AstraZeneca Pharmaceuticals LP
Wilmington, DE 19850
QTERN is a registered trademark of the AstraZeneca group of companies.
-
MEDICATION GUIDE
MEDICATION GUIDE
QTERN® [CUE-turn]
(dapagliflozin and saxagliptin)
tablets, for oral use
What is the most important information I should know about QTERN?
Serious side effects can happen to people taking QTERN, including:
- •
- • Diabetic Ketoacidosis (increased ketones in your blood or urine) in people with type 1 diabetes and other ketoacidosis. QTERN can cause ketoacidosis that can be life-threatening and may lead to death. Ketoacidosis is a serious condition which needs to be treated in a hospital. People with type 1 diabetes have a high risk of getting ketoacidosis. People with type 2 diabetes or pancreas problems also have an increased risk of getting ketoacidosis. Ketoacidosis can also happen in people who are sick, cannot eat or drink as usual, skip meals, are on a diet high in fat and low in carbohydrates (ketogenic diet), take less than the usual amount of insulin or miss insulin doses, drink too much alcohol, have a loss of too much fluid from the body (volume depletion), or who have surgery. Ketoacidosis can happen even if your blood sugar is less than 250 mg/dL. Your healthcare provider may ask you to periodically check ketones in your urine or blood.
- Stop taking QTERN and call your healthcare provider or get medical help right away if you get any of the following. If possible, check for ketones in your urine or blood, even if your blood sugar is less than 250 mg/dL.
- ∘
- nausea
- ∘
- vomiting
- ∘
- stomach area (abdominal) pain
- ∘
- tiredness
- ∘
- trouble breathing
- ∘
- ketones in your urine or blood
- •
-
Inflammation of the pancreas (pancreatitis). Saxagliptin, one of the medicines in QTERN, can cause inflammation of the pancreas, which may be severe and lead to death. Certain medical problems make you more likely to get pancreatitis.
Before you start taking QTERN, tell your healthcare provider if you have ever had:- ∘
- inflammation of your pancreas (pancreatitis)
- ∘
- stones in your gallbladder (gallstones)
- ∘
- a history of alcoholism
- ∘
- high blood triglyceride levels
- It is not known if having these medical problems will make you more likely to get pancreatitis with QTERN.
Stop taking QTERN and contact your healthcare provider right away if you have pain in your stomach area (abdomen) that is severe and will not go away. The pain may be felt going from your abdomen through to your back. The pain may happen with or without vomiting. These may be symptoms of pancreatitis. - •
-
Heart failure. Heart failure means your heart does not pump blood well enough.
Before you start taking QTERN, tell your healthcare provider if you have ever had heart failure or have problems with your kidneys.
Contact your healthcare provider right away if you have any of the following symptoms:- ∘
- increasing shortness of breath or trouble breathing, especially when you lie down
- ∘
- swelling or fluid retention, especially in the feet, ankles, or legs
- ∘
- an unusually fast increase in weight
- ∘
- unusual tiredness
- These may be symptoms of heart failure.
- •
-
Dehydration. QTERN can cause some people to become dehydrated (the loss of body water and salt). Dehydration may cause you to feel dizzy, faint, lightheaded, or weak, especially when you stand up (orthostatic hypotension). There have been reports of sudden kidney injury in people with Type 2 diabetes who are taking dapagliflozin, a component of QTERN. You may be at a higher risk of dehydration if you:
- ∘
- take medicines to lower your blood pressure, including water pills (diuretics)
- ∘
- are on a low salt diet
- ∘
- have kidney problems
- ∘
- are 65 years of age or older
- Talk to your healthcare provider about what you can do to prevent dehydration including how much fluid you should drink on a daily basis. Call your healthcare provider right away if you reduce the amount of food or liquid you drink, for example if you cannot eat or you start to lose liquids from your body, for example from vomiting, diarrhea, or being in the sun too long.
What is QTERN?
QTERN is a prescription medicine that contains dapagliflozin and saxagliptin. QTERN is used along with diet and exercise to improve blood sugar (glucose) control in adults with type 2 diabetes.
- •
- QTERN is not for use to improve blood sugar (glucose) control in people with type 1 diabetes.
- •
- It is not known if QTERN is safe and effective in children younger than 18 years of age.
Who should not take QTERN?
Do not take QTERN if you:
- •
- are allergic to dapagliflozin, saxagliptin, or any of the ingredients in QTERN. See the end of this Medication Guide for a list of ingredients in QTERN.
Symptoms of a serious allergic reaction to QTERN may include:- ∘
- swelling of the face, lips, throat and other areas of your skin
- ∘
- difficulty with swallowing or breathing
- ∘
- rash, itching, flaking, or peeling
- ∘
- raised red areas on your skin (hives)
- If you have any of these symptoms, stop taking QTERN and contact your healthcare provider or go to the nearest hospital emergency room right away.
- •
- have moderate to severe kidney problems or are on dialysis.
Before taking QTERN, tell your healthcare provider about all of your medical conditions, including if you:
- •
- have type 1 diabetes or have had diabetic ketoacidosis (increased ketones in your blood or urine).
- •
- have a decrease in your insulin dose.
- •
- have a serious infection.
- •
- have a history of infection of the vagina or penis.
- •
- have kidney problems.
- •
- have liver problems.
- •
- have a history of urinary tract infections or problems with urination.
- •
- are on a low sodium (salt) diet. Your healthcare provider may ask you to change your diet.
- •
- are going to have surgery. Your healthcare provider may stop your QTERN before you have surgery. Talk to your healthcare provider if you are having surgery about when to stop taking QTERN and when to start it again.
- •
- are eating less or there is a change in your diet.
- •
- are dehydrated.
- •
- have or have had problems with your pancreas, including pancreatitis or surgery on your pancreas.
- •
- drink alcohol very often or drink a lot of alcohol in the short term (“binge” drinking).
- •
- have heart problems, including heart failure.
- •
- have had a history of swelling of the face, lips, tongue and throat (angioedema) when you have taken a dipeptidyl peptidase 4 (DPP-4) inhibitor like saxagliptin one of the medicines in QTERN. If you are not sure that you have taken this medicine before, ask your healthcare provider.
- •
- are pregnant or plan to become pregnant. QTERN may harm your unborn baby. If you are pregnant or plan to become pregnant, talk to your healthcare provider about the best way to control your blood sugar.
- •
- are breastfeeding or plan to breastfeed. It is not known if QTERN passes into your breast milk. Talk with your healthcare provider about the best way to feed your baby if you are taking QTERN. Breastfeeding is not recommended while taking QTERN.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
QTERN may affect the way other medicines work, and other medicines may affect how QTERN works. Contact your healthcare provider if you will be starting or stopping certain other types of medicines such as antibiotics or medicines that treat fungus or HIV/AIDS, because your dose of QTERN might need to be changed.
Know the medicines you take. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take QTERN?
- •
- Take QTERN exactly as your healthcare provider tells you to take it.
- •
- Take QTERN by mouth 1 time each day in the morning with or without food.
- •
- Your healthcare provider will tell you how much QTERN to take and when to take it. Your healthcare provider may change your dose if needed.
- •
- Swallow QTERN whole. Do not crush, cut or chew QTERN tablets.
- •
- If you miss a dose, take it as soon as you remember. If it is almost time for your next dose, skip the missed dose and take the medicine at the next regularly scheduled time. Do not take 2 doses of QTERN at the same time unless your healthcare provider tells you to do so. Talk to your healthcare provider if you have questions about a missed dose.
- •
- If you take too much QTERN, call your healthcare provider, or go to the nearest hospital emergency room right away.
- •
- During periods of stress on the body, such as fever, trauma, infection, or surgery, the amount of diabetes medicine you need may change. Tell your healthcare provider right away if you have any of these conditions and follow your healthcare provider’s instructions.
- •
- Your healthcare provider may tell you to take QTERN along with other diabetes medicines. Low blood sugar can happen more often when QTERN is taken with certain other diabetes medicines. See “What are the possible side effects of QTERN?”.
- •
- QTERN will cause your urine to test positive for glucose.
- •
- Your healthcare provider may do certain blood tests before you start QTERN and during treatment as needed. Your healthcare provider may change your dose of QTERN based on the results of your blood tests.
What are the possible side effects of QTERN?
QTERN may cause serious side effects, including:
- •
- See “What is the most important information I should know about QTERN?”.
- •
- Serious urinary tract infections. Serious urinary tract infections that may lead to hospitalization have happened in people who are taking dapagliflozin. Tell your healthcare provider if you have any signs or symptoms of a urinary tract infection such as a burning feeling when passing urine, a need to urinate often, the need to urinate right away, pain in the lower part of your stomach (pelvis), or blood in the urine. Sometimes people also may have a fever, back pain, nausea or vomiting.
- •
- Low blood sugar (hypoglycemia). If you take QTERN with another medicine that can cause low blood sugar, such as a sulfonylurea or insulin, this can increase your risk of getting low blood sugar. Tell your healthcare provider if you take other diabetes medicines. Signs and symptoms of low blood sugar may include:
- ∘
- headache
- ∘
- confusion
- ∘
- hunger
- ∘
- shaking or feeling jittery
- ∘
- drowsiness
- ∘
- dizziness
- ∘
- fast heartbeat
- ∘
- weakness
- ∘
- sweating
- ∘
- irritability
- •
- A rare but serious bacterial infection that causes damage to the tissue under the skin (necrotizing fasciitis) in the area between and around the anus and genitals (perineum). Necrotizing fasciitis of the perineum has happened in women and men who take dapagliflozin, one of the medicines in QTERN. Necrotizing fasciitis of the perineum may lead to hospitalization, may require multiple surgeries and may lead to death. Seek medical attention immediately if you have fever or you are feeling very weak, tired or uncomfortable (malaise) and you develop any of the following symptoms in the area between and around the anus and genitals:
-
- ∘
- pain or tenderness
-
- ∘
- swelling
-
- ∘
- redness of skin (erythema)
- •
- Serious allergic reaction. If you have any symptoms of a serious allergic reactions, stop taking QTERN and call your healthcare provider right away or go to the nearest hospital emergency room. See “Who should not take QTERN?”. Your healthcare provider may give you a medicine for your allergic reaction and prescribe a different medicine for your diabetes.
- •
-
Vaginal yeast infection. Women who take QTERN may get vaginal yeast infections. Symptoms of a vaginal yeast infection include:
- ∘
- vaginal odor
- ∘
- white or yellowish vaginal discharge (discharge may be lumpy or look like cottage cheese)
- ∘
- vaginal itching
- •
-
Yeast infection of the penis (balanitis). Swelling of an uncircumcised penis may develop that makes it difficult to pull back the skin around the tip of the penis. Other symptoms of yeast infection of the penis include:
- ∘
- redness, itching, or swelling of the penis
- ∘
- foul smelling discharge from the penis
- ∘
- rash of the penis
- ∘
- pain in the skin around the penis
Talk to your healthcare provider about what to do if you get symptoms of a yeast infection of the vagina or penis. Your healthcare provider may suggest you use an over-the-counter antifungal medicine. Talk to your healthcare provider right away if you use an over-the-counter antifungal medicine and your symptoms do not go away.
- •
- Joint pain. Some people who take DPP-4 inhibitors like saxagliptin, one of the medicines in QTERN, may develop joint pain that can be severe. Call your healthcare provider right away if you have severe joint pain.
- •
- Skin reaction. Some people who take DPP-4 inhibitors like saxagliptin, one of the medicines in QTERN, may develop a skin reaction called bullous pemphigoid that can require treatment in a hospital. Tell your healthcare provider right away if you develop blisters or the breakdown of the outer layer of your skin (erosion). Your healthcare provider may tell you to stop taking QTERN.
The most common side effects of QTERN include:
- •
- upper respiratory tract infection
- •
- urinary tract infection
- •
- abnormal amounts of fats in the blood (dyslipidemia)
These are not all of the possible side effects of QTERN.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store QTERN?
Store QTERN at room temperature between 68○F and 77○F (20○C and 25○C).
Keep QTERN and all medicines out of the reach of children.
General information about the safe and effective use of QTERN.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use QTERN for a condition for which it was not prescribed. Do not give QTERN to other people, even if they have the same symptoms you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about QTERN that is written for health professionals.
What are the ingredients in QTERN?
Active ingredients: dapagliflozin and saxagliptin
Inactive ingredients: anhydrous lactose, croscarmellose sodium, iron oxides, magnesium stearate, microcrystalline cellulose, polyvinyl alcohol, polyethylene glycol, silicon dioxide, talc, titanium dioxide; hydrochloric acid and sodium hydroxide as needed.
QTERN is a registered trademark of the AstraZeneca group of companies.
Distributed by:
AstraZeneca Pharmaceuticals LP
Wilmington, DE 19850
For more information about QTERN, go to www.QTERN.com or call 1-800-236-9933.
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 09/2023
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 5 mg/5 mg
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 10 mg/5 mg
-
INGREDIENTS AND APPEARANCE
QTERN
dapagliflozin and saxagliptin tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0310-6780 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DAPAGLIFLOZIN (UNII: 1ULL0QJ8UC) (DAPAGLIFLOZIN - UNII:1ULL0QJ8UC) DAPAGLIFLOZIN 10 mg SAXAGLIPTIN HYDROCHLORIDE (UNII: Z8J84YIX6L) (SAXAGLIPTIN ANHYDROUS - UNII:8I7IO46IVQ) SAXAGLIPTIN ANHYDROUS 5 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FERROUS OXIDE (UNII: G7036X8B5H) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color BROWN Score no score Shape ROUND (biconvex) Size 8mm Flavor Imprint Code 5;10;1122 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0310-6780-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 12/04/2017 12/31/2025 2 NDC:0310-6780-95 7 in 1 BLISTER PACK; Type 0: Not a Combination Product 12/04/2017 07/31/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209091 12/04/2017 12/31/2025 QTERN
dapagliflozin and saxagliptin tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0310-6770 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DAPAGLIFLOZIN (UNII: 1ULL0QJ8UC) (DAPAGLIFLOZIN - UNII:1ULL0QJ8UC) DAPAGLIFLOZIN 5 mg SAXAGLIPTIN HYDROCHLORIDE (UNII: Z8J84YIX6L) (SAXAGLIPTIN ANHYDROUS - UNII:8I7IO46IVQ) SAXAGLIPTIN ANHYDROUS 5 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FERROUS OXIDE (UNII: G7036X8B5H) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color PURPLE (light reddish) Score no score Shape ROUND (biconvex) Size 8mm Flavor Imprint Code 5;5;1120 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0310-6770-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 06/17/2019 02/28/2026 2 NDC:0310-6770-95 7 in 1 BLISTER PACK; Type 0: Not a Combination Product 07/01/2019 07/31/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209091 12/04/2017 02/28/2026 Labeler - AstraZeneca Pharmaceuticals LP (054743190) Registrant - AstraZeneca PLC (230790719)