Label: ACETIC ACID irrigant
- NDC Code(s): 0338-0656-04
- Packager: Baxter Healthcare Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 2, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
0.25% Acetic Acid Irrigation, USP, is a sterile, nonpyrogenic hypotonic solution for irrigation of the urinary bladder. Each 100 mL contains 250 mg Glacial Acetic Acid, USP, (CH3COOH) in Water for Injection, USP. pH 3.0 (2.8 to 3.4). Osmolarity: 42 mOsmol/L (calc.). No antimicrobial agent has been added.
The container is made from specially formulated polyolefin (PL 325). The polyolefin is a copolymer of ethylene and propylene. It contains no plasticizers or other mobile additives. As a result, the container has virtually no extractability or leachability. The total extractables after two years of storage being less than 0.01 ppm. It is also relatively impermeable to water vapor transmission and, therefore, requires no vapor barrier to maintain the proper drug concentration.
- CLINICAL PHARMACOLOGY
-
INDICATIONS AND USAGE
0.25% Acetic Acid Irrigation, USP, is indicated as a constant or intermittent bladder rinse to help prevent the growth and proliferation of susceptible urinary pathogens (especially ammonia forming bacteria) in the management of patients who require prolonged placement of an indwelling urethral catheter. It also may be used for periodic irrigation of an indwelling catheter to help maintain patency by reducing the formation of calcium encrustations.
- CONTRAINDICATIONS
-
WARNINGS
Not for injection.
Use of this solution in patients with mucosal lesions of the urinary bladder may be harmful due to irritation of the lesion. Absorption via open lesions of the bladder mucosa may result in systemic acidosis.
The contents of an opened container should be used promptly to minimize the possibility of bacterial growth or pyrogen formation. Discard the unused portion of irrigating solution since no antimicrobial agent has been added.
-
PRECAUTIONS
Pregnancy
Teratogenic Effects
Animal reproduction studies have not been conducted with 0.25% Acetic Acid Irrigation, USP. It is also not known whether 0.25% Acetic Acid Irrigation, USP can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. 0.25% Acetic Acid Irrigation, USP should be given to a pregnant woman only if clearly needed.
- ADVERSE REACTIONS
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
0.25% Acetic Acid Irrigation, USP is supplied in a plastic pour bottle as follows:
2F7184
1000 mL
NDC 0338-0656-04
Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. It is recommended that the product be stored at room temperature (25°C): brief exposure up to 40ºC does not adversely affect the product.
- SPL UNCLASSIFIED SECTION
-
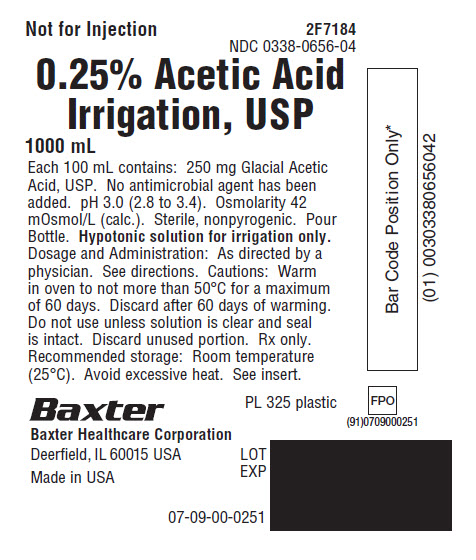
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL
Not for Injection 2F7184
NDC 0338-0656-040.25% Acetic Acid
Irrigation, USP1000 mL
Each 100 mL contains: 250 mg Glacial Acetic
Acid, USP. No antimicrobial agent has been
added. pH 3.0 (2.8 to 3.4). Osmolarity 42
mOsmol/L (calc.). Sterile, nonpyrogenic. Pour
Bottle. Hypotonic solution for irrigation only.
Dosage and Administration: As directed by a
physician. See directions. Cautions: Warm
in oven to not more than 50°C for a maximum
of 60 days. Discard after 60 days of warming.
Do not use unless solution is clear and seal
is intact. Discard unused portion. Rx only.
Recommended storage: Room temperature
(25°C). Avoid excessive heat. See insert.PL 325 plastic
Baxter Logo
Baxter Healthcare Corporation
Deerfield, IL 60015 USAMade in USA
LOT
EXP07-09-00-0251
FPO
(91)0709000251
Bar CodeBar Code Posit ion Only*
(01) 00303380656042 -
INGREDIENTS AND APPEARANCE
ACETIC ACID
acetic acid irrigantProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0338-0656 Route of Administration IRRIGATION Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETIC ACID (UNII: Q40Q9N063P) (ACETIC ACID - UNII:Q40Q9N063P) ACETIC ACID 250 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-0656-04 1000 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/19/1982 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018523 02/19/1982 Labeler - Baxter Healthcare Corporation (005083209) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Corporation 001728059 MANUFACTURE(0338-0656) , ANALYSIS(0338-0656) , PACK(0338-0656) , LABEL(0338-0656) , STERILIZE(0338-0656) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Corporation 194684502 ANALYSIS(0338-0656)