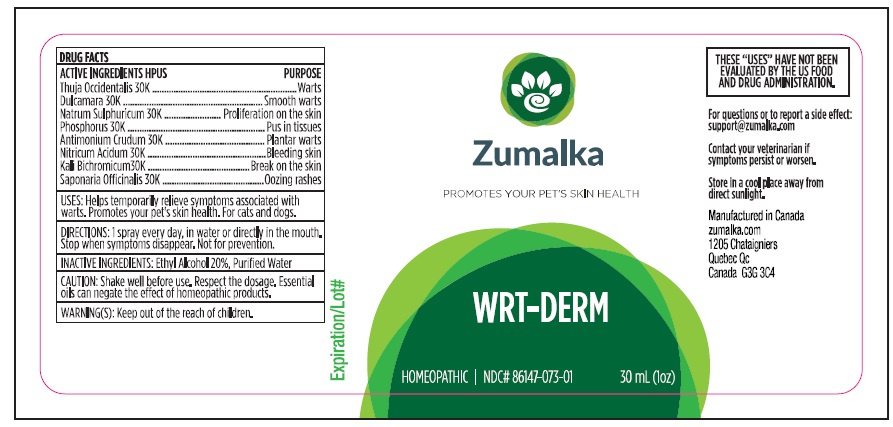
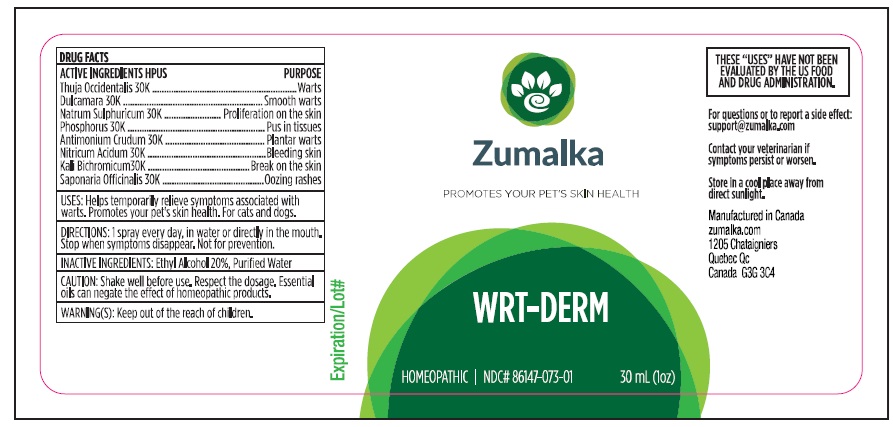
Label: WRT-DERM- thuja occidentalis leafy twig, solanum dulcamara top, potassium dichromate, sodium sulfate, phosphorus, antimony trisulfide, nitric acid, saponaria officinalis root, solidago virgaurea flowering top liquid
- NDC Code(s): 86147-073-01
- Packager: Groupe Cyrenne Inc.
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- PURPOSE
- Uses
- Warnings
- KEEP OUT OF REACH OF CHILDREN
- Direction
- Inactive ingredients
- Other information
- Product label
-
INGREDIENTS AND APPEARANCE
WRT-DERM
thuja occidentalis leafy twig, solanum dulcamara top, potassium dichromate, sodium sulfate, phosphorus, antimony trisulfide, nitric acid, saponaria officinalis root, solidago virgaurea flowering top liquidProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:86147-073 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength THUJA OCCIDENTALIS LEAFY TWIG (UNII: 1NT28V9397) (THUJA OCCIDENTALIS LEAFY TWIG - UNII:1NT28V9397) THUJA OCCIDENTALIS LEAFY TWIG 30 [kp_C] in 30 mL SOLANUM DULCAMARA TOP (UNII: KPS1B1162N) (SOLANUM DULCAMARA TOP - UNII:KPS1B1162N) SOLANUM DULCAMARA TOP 30 [kp_C] in 30 mL POTASSIUM DICHROMATE (UNII: T4423S18FM) (DICHROMATE ION - UNII:9LKY4BFN2V) POTASSIUM DICHROMATE 30 [kp_C] in 30 mL SODIUM SULFATE (UNII: 0YPR65R21J) (SODIUM SULFATE ANHYDROUS - UNII:36KCS0R750) SODIUM SULFATE 30 [kp_C] in 30 mL PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 30 [kp_C] in 30 mL ANTIMONY TRISULFIDE (UNII: F79059A38U) (ANTIMONY TRISULFIDE - UNII:F79059A38U) ANTIMONY TRISULFIDE 30 [kp_C] in 30 mL NITRIC ACID (UNII: 411VRN1TV4) (NITRIC ACID - UNII:411VRN1TV4) NITRIC ACID 30 [kp_C] in 30 mL SAPONARIA OFFICINALIS ROOT (UNII: RI2K1BMA8B) (SAPONARIA OFFICINALIS ROOT - UNII:RI2K1BMA8B) SAPONARIA OFFICINALIS ROOT 30 [kp_C] in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:86147-073-01 30 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 10/14/2021 Labeler - Groupe Cyrenne Inc. (208482650) Establishment Name Address ID/FEI Business Operations Laboratoire Schmidt-Nagel Inc 203869883 manufacture, api manufacture