Label: REVLON COLORSTAY LIQUID MAKEUP FOR NORMAL TO DRY SKIN- zinc oxide, titanium dioxide make-up liquid
- NDC Code(s): 10967-643-01
- Packager: Revlon Consumer Products Corp
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 21, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients:
- Warnings:
-
Inactive Ingredients:
Cyclopentasiloxane, Aqua/Water/Eau, Dimethicone, Trimethylsiloxysilicate, PEG-9 Polydimethylsiloxyethyl Dimethicone, Phenyl Trimethicone, Methyl Methacrylate Crosspolymer, Silica, Butylene Glycol, Nylon-12, Dimethicone/PEG-10/15 Crosspolymer, Alumina, Disteardimonium Hectorite, Ethylene Brassylate, Hydrogen Dimethicone, Hydrolyzed Vegetable Protein, Laureth-7, Magnesium Sulfate, Methicone, Mica, Polyglyceryl-3 Diisostearate, Salicylic Acid, Sodium Hyaluronate, Tetrasodium EDTA, Tocopheryl Acetate, Tribehenin, Triethoxycaprylylsilane, Triethyl Citrate, Ethylparaben, Methylparaben, Phenoxyethanol.
MAY CONTAIN:
[Iron Oxides (CI 77491), Iron Oxides (CI 77492), Iron Oxides (CI 77499), Titanium Dioxide (CI 77891)] B02217
- Purpose
- To Use
- Front and Back Label_Revlon Colorstay Normal to Dry Foundation
-
INGREDIENTS AND APPEARANCE
REVLON COLORSTAY LIQUID MAKEUP FOR NORMAL TO DRY SKIN
zinc oxide, titanium dioxide make-up liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10967-643 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 2.1 mg in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 4.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) METHICONE (20 CST) (UNII: 6777U11MKT) LAURETH-7 (UNII: Z95S6G8201) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYLPARABEN (UNII: A2I8C7HI9T) ETHYLPARABEN (UNII: 14255EXE39) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) TRIBEHENIN (UNII: 8OC9U7TQZ0) NYLON-12 (UNII: 446U8J075B) ALUMINUM OXIDE (UNII: LMI26O6933) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) ETHYLENE BRASSYLATE (UNII: 9A87HC7ROD) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) EDETATE SODIUM (UNII: MP1J8420LU) LEVOMENOL (UNII: 24WE03BX2T) ETHYLHEXYL PALMITATE (UNII: 2865993309) TOCOPHEROL (UNII: R0ZB2556P8) SODIUM CITRATE (UNII: 1Q73Q2JULR) DIPROPYLENE GLYCOL (UNII: E107L85C40) SALICYLIC ACID (UNII: O414PZ4LPZ) MALVA SYLVESTRIS FLOWERING TOP (UNII: X1U1U0N90J) LILIUM CANDIDUM BULB (UNII: AHG15J8AM0) CYMBIDIUM HOOKERIANUM FLOWER (UNII: H2DJ03DI5I) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PEG-12 GLYCERYL DIMYRISTATE (UNII: VS4W16AQ3X) CYCLOMETHICONE 6 (UNII: XHK3U310BA) POLYSORBATE 20 (UNII: 7T1F30V5YH) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10967-643-01 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 06/01/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 06/01/2012 Labeler - Revlon Consumer Products Corp (788820165) Establishment Name Address ID/FEI Business Operations REVLON, INC. 809725570 manufacture(10967-643)

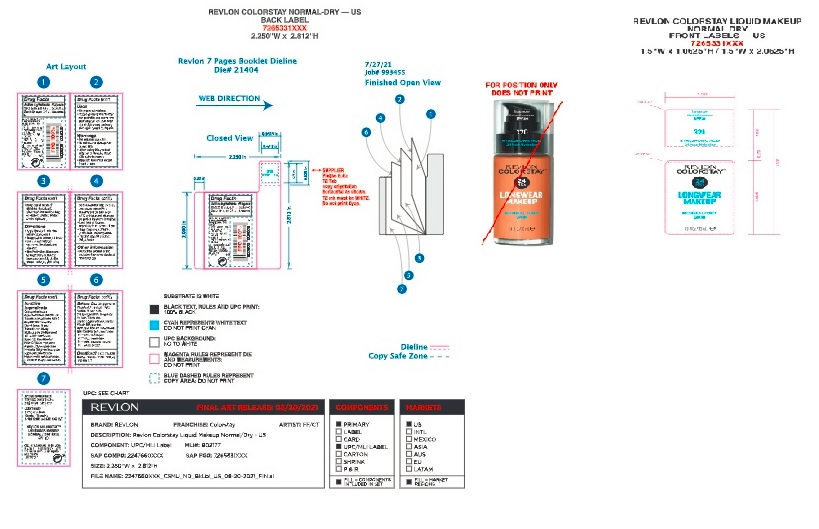
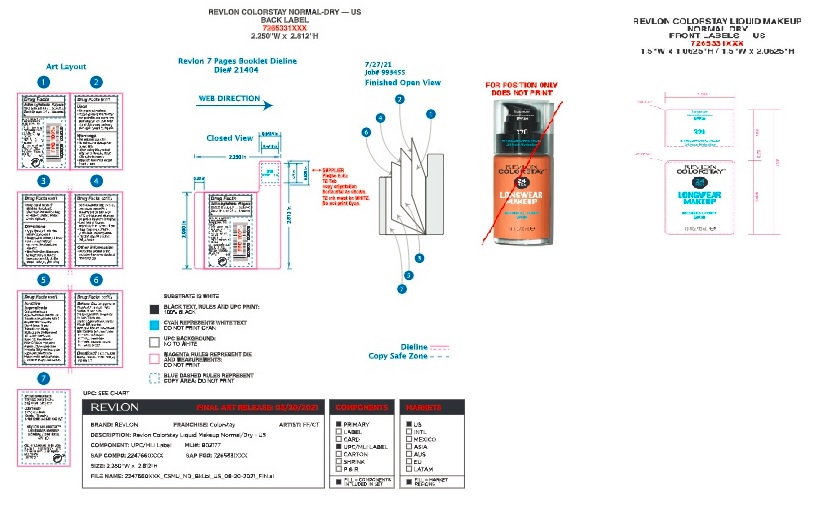 Front and Back Label_
Front and Back Label_