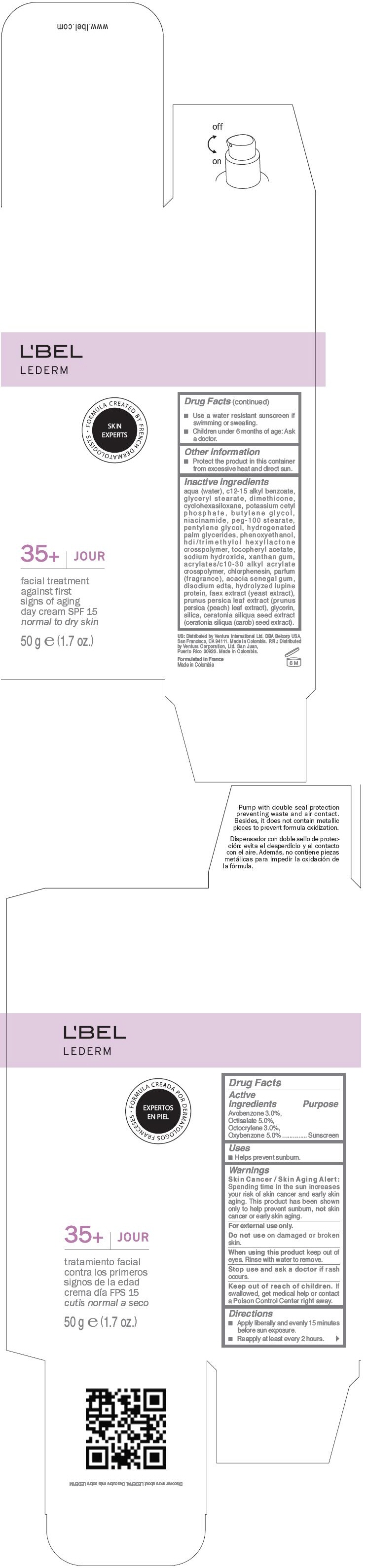
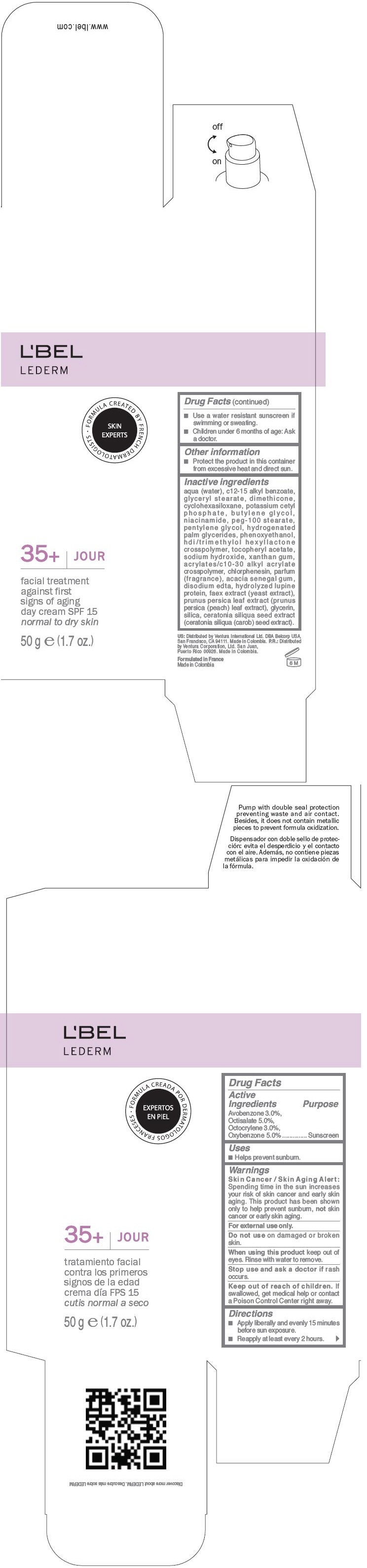
Label: LBEL LEDERM 35PLUS JOUR SPF 15 FACIAL TREATMENT AGAINST FIRST SIGNS OF AGING DAY - NORMAL TO DRY SKIN- avobenzone, octisalate, octocrylene, and oxybenzone cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 13537-445-01, 13537-445-03, 13537-445-04, 13537-445-05, view more13537-445-06 - Packager: Ventura Corporation LTD.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 1, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
AQUA (WATER), C12-15 ALKYL BENZOATE, GLYCERYL STEARATE, DIMETHICONE, CYCLOHEXASILOXANE, POTASSIUM CETYL PHOSPHATE, BUTYLENE GLYCOL, NIACINAMIDE, PEG-100 STEARATE, PENTYLENE GLYCOL, HYDROGENATED PALM GLYCERIDES, PHENOXYETHANOL, HDI/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER, TOCOPHERYL ACETATE, SODIUM HYDROXIDE, XANTHAN GUM, ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER, CHLORPHENESIN, PARFUM (FRAGRANCE), ACACIA SENEGAL GUM, DISODIUM EDTA, HYDROLYZED LUPINE PROTEIN, FAEX EXTRACT (YEAST EXTRACT), PRUNUS PERSICA LEAF EXTRACT (PRUNUS PERSICA (PEACH) LEAF EXTRACT), GLYCERIN, SILICA, CERATONIA SILIQUA SEED EXTRACT (CERATONIA SILIQUA (CAROB) SEED EXTRACT)
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 50 g Bottle Carton
-
INGREDIENTS AND APPEARANCE
LBEL LEDERM 35PLUS JOUR SPF 15 FACIAL TREATMENT AGAINST FIRST SIGNS OF AGING DAY - NORMAL TO DRY SKIN
avobenzone, octisalate, octocrylene, and oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-445 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 0.03 g in 1 g Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 0.05 g in 1 g Octocrylene (UNII: 5A68WGF6WM) (Octocrylene - UNII:5A68WGF6WM) Octocrylene 0.03 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.05 g in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) DIMETHICONE (UNII: 92RU3N3Y1O) CYCLOMETHICONE 6 (UNII: XHK3U310BA) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) NIACINAMIDE (UNII: 25X51I8RD4) PEG-100 STEARATE (UNII: YD01N1999R) PENTYLENE GLYCOL (UNII: 50C1307PZG) HYDROGENATED PALM GLYCERIDES (UNII: YCZ8EM144Q) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SODIUM HYDROXIDE (UNII: 55X04QC32I) XANTHAN GUM (UNII: TTV12P4NEE) CHLORPHENESIN (UNII: I670DAL4SZ) ACACIA (UNII: 5C5403N26O) EDETATE DISODIUM (UNII: 7FLD91C86K) YEAST (UNII: 3NY3SM6B8U) PRUNUS PERSICA LEAF (UNII: VN3501T41P) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CAROB (UNII: 5MG5Z946UO) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-445-01 1 g in 1 PACKET 2 NDC:13537-445-04 1 in 1 BOX 2 NDC:13537-445-03 5 g in 1 BOTTLE, PLASTIC 3 NDC:13537-445-06 1 in 1 BOX 3 NDC:13537-445-05 50 g in 1 BOTTLE, GLASS Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 04/01/2013 Labeler - Ventura Corporation LTD. (602751344) Establishment Name Address ID/FEI Business Operations Bel Star S.A. (Colombia) 880160197 MANUFACTURE(13537-445) Establishment Name Address ID/FEI Business Operations Yobel Supply Chain Management S.A. (Peru) 934116930 MANUFACTURE(13537-445)