Label: FEXOFENADINE HYDROCHLORIDE tablet, film coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 68554-5061-0, 68554-5061-1, 68554-5061-2, 68554-5061-3, view more68554-5061-4, 68554-5061-5, 68554-5061-6, 68554-5062-0, 68554-5062-1, 68554-5062-2, 68554-5062-3, 68554-5062-4, 68554-5062-5, 68554-5062-6, 68554-5063-0, 68554-5063-1, 68554-5063-2, 68554-5063-3, 68554-5063-4, 68554-5063-5, 68554-5063-6 - Packager: Hetero Labs Limited
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated September 8, 2016
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
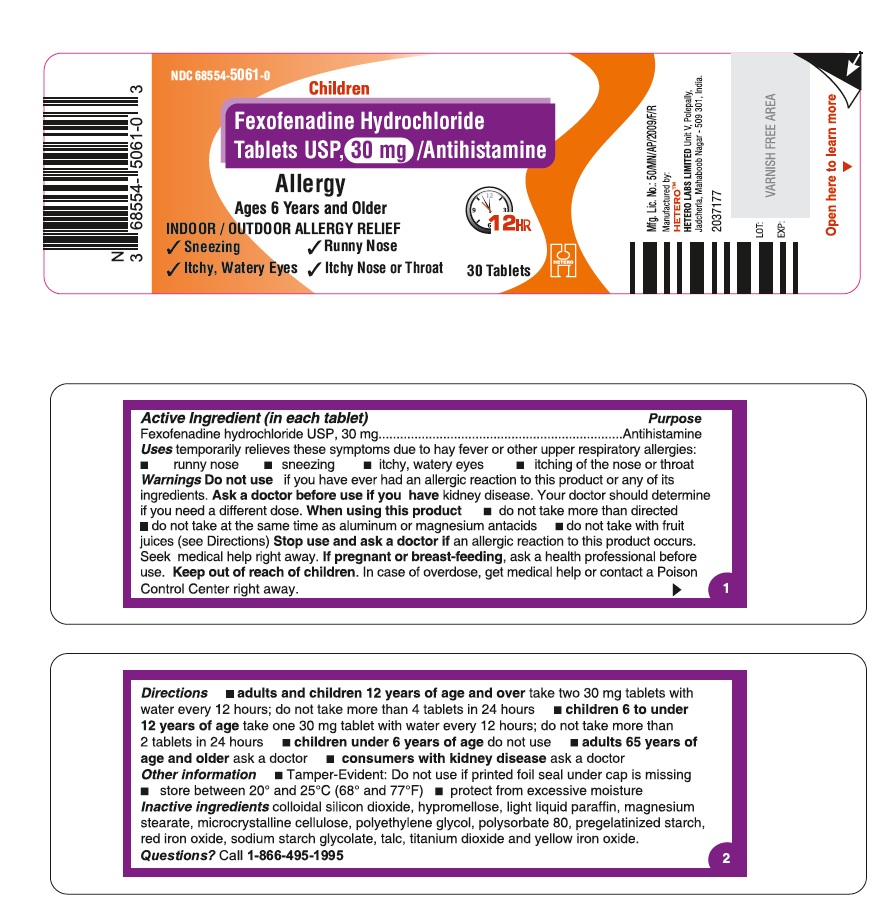
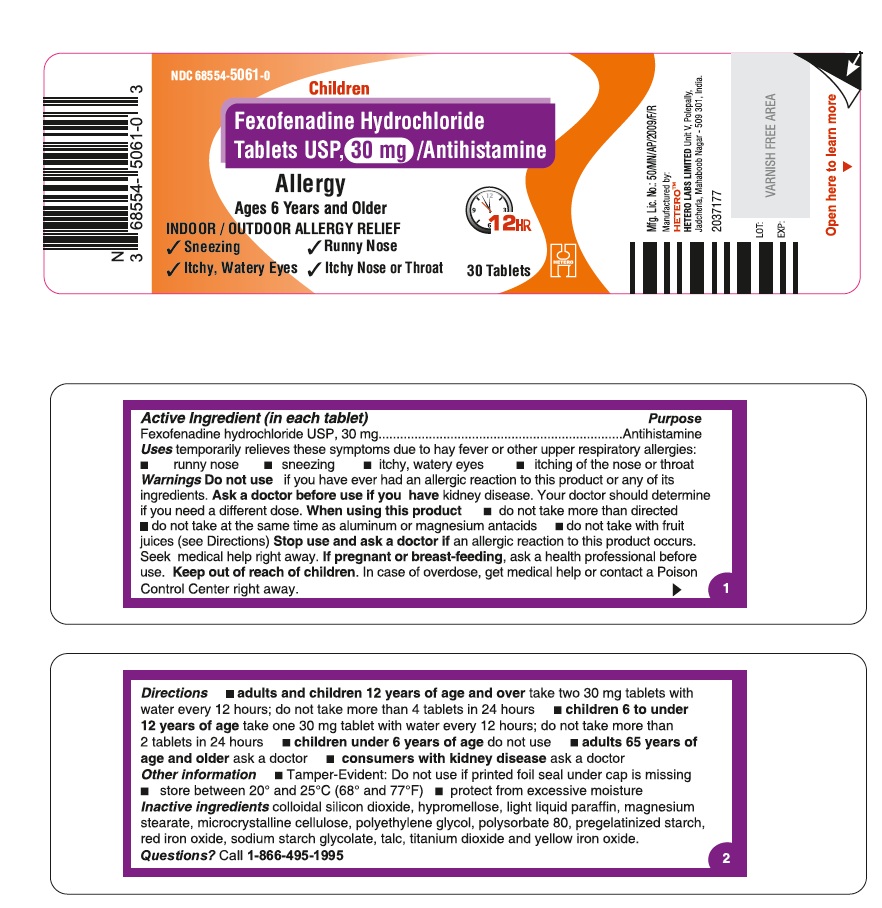
- ACTIVE INGREDIENT(S)
- PURPOSE
- USE(S)
- WARNINGS
- DO NOT USE
- ASK A DOCTOR BEFORE USE IF
- WHEN USING THIS PRODUCT
- STOP USE AND ASK DOCTOR IF
- PREGNANCY/BREASTFEEDING
- KEEP OUT OF REACH OF CHILDREN
-
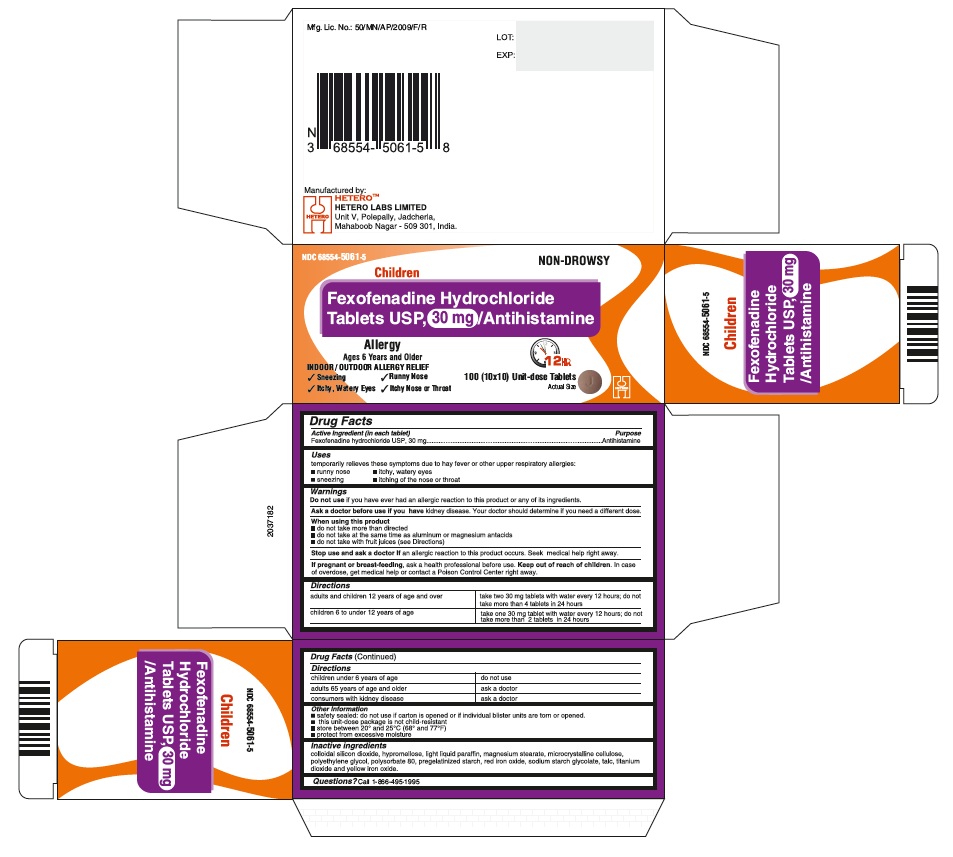
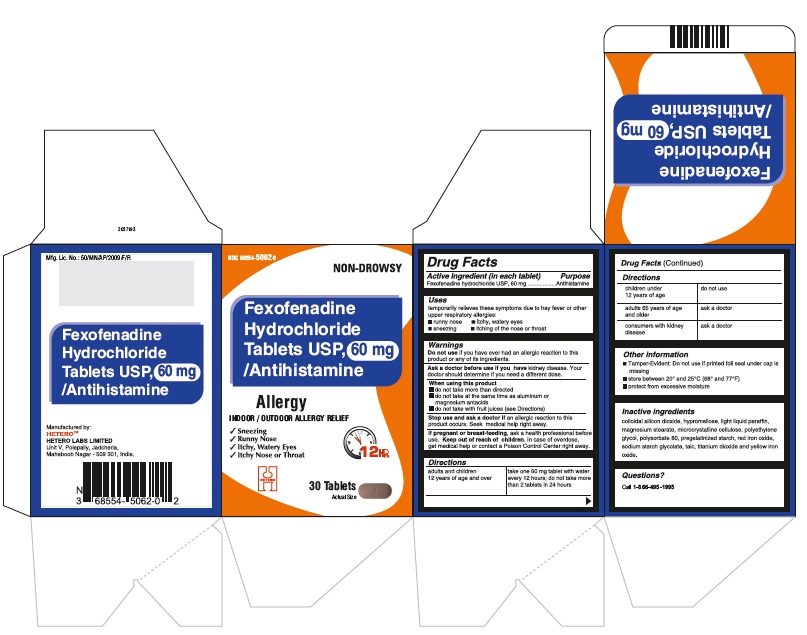
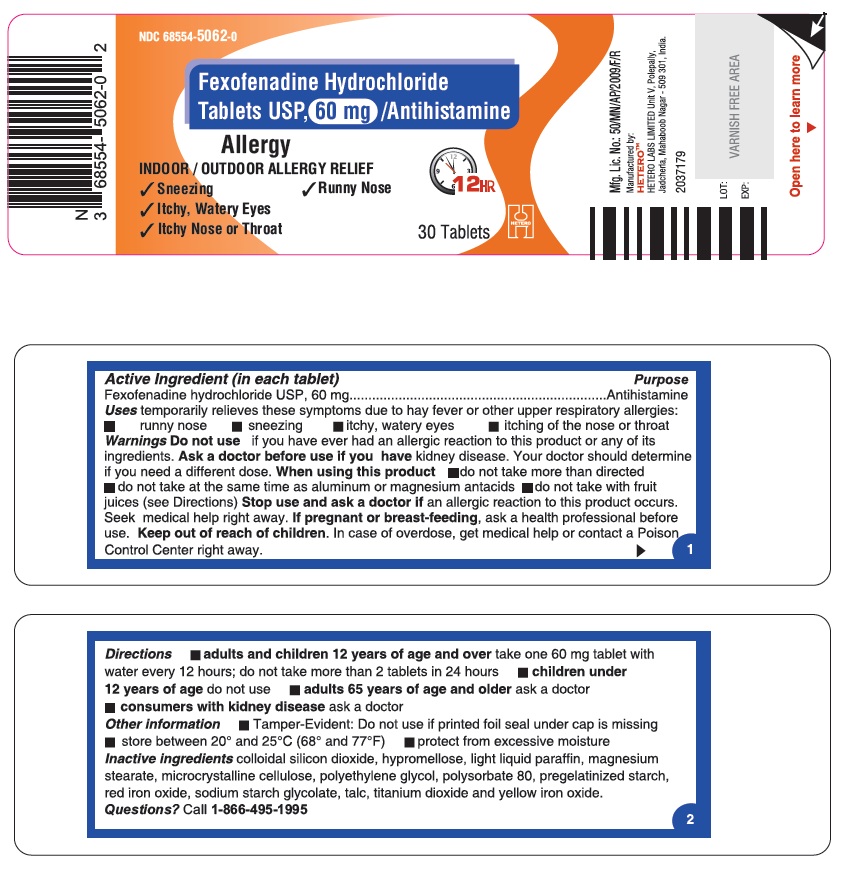
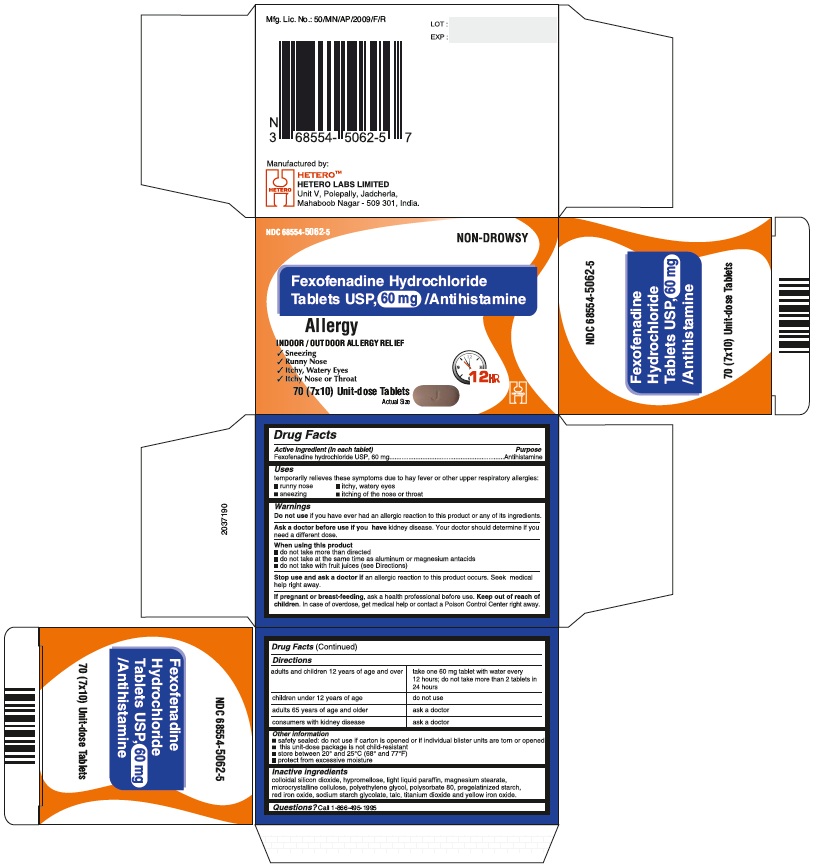
DIRECTIONS
30 mg
adults and children 12 years of age and over
take two 30 mg tablets with water every 12 hours; do not take more than 4 tablets in 24 hours
children 6 to under 12 years of age
take one 30 mg tablet with water every 12 hours; do not take more than 2 tablets in 24 hours
children under 6 years of age
do not use
adults 65 years of age and older
ask a doctor
consumers with kidney disease
ask a doctor
60 mg
adults and children 12 years of age and over
take one 60 mg tablet with water every 12 hours; do not take more than 2 tablets in 24 hours
children under 12 years of age
do not use
adults 65 years of age and older
ask a doctor
consumers with kidney disease
ask a doctor
180 mg
adults and children 12 years of age and over
take one 180 mg tablet with water once a day; do not take more than 1 tablet in 24 hours
children under 12 years of age
do not use
adults 65 years of age and older
ask a doctor
consumers with kidney disease
ask a doctor
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- QUESTIONS?
-
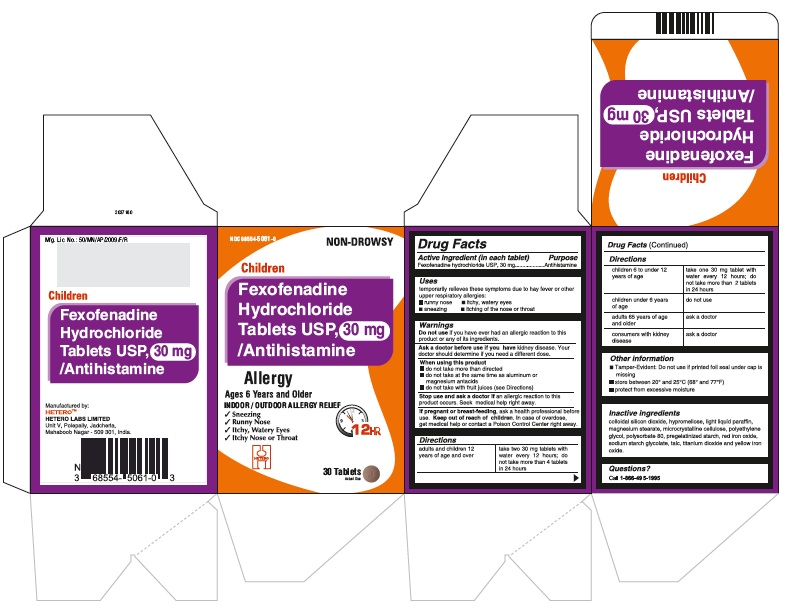
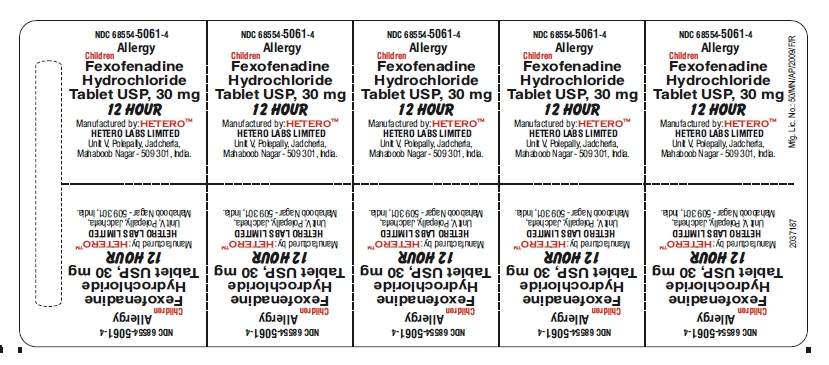
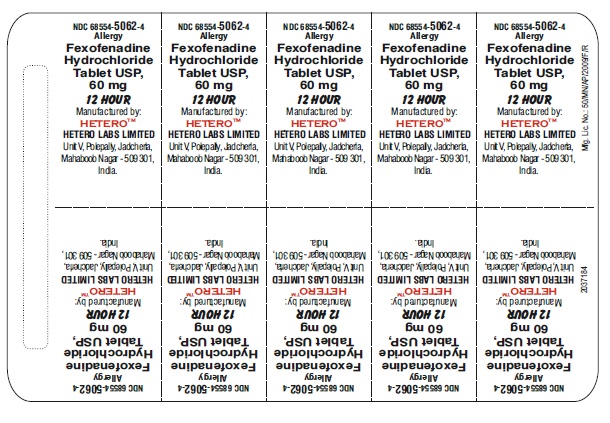
PRINCIPAL DISPLAY PANEL
Fexofenadine Hydrochloride Tablets USP, 30 mg - Container Carton
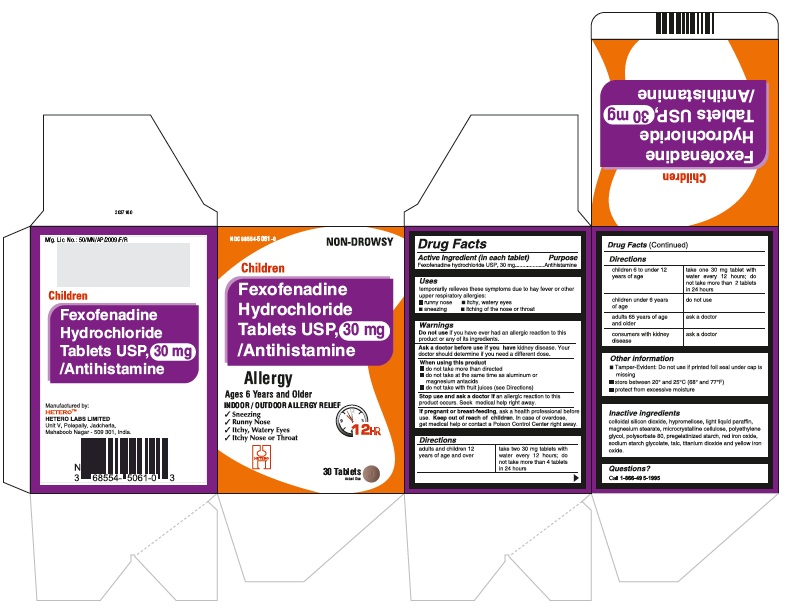
Fexofenadine Hydrochloride Tablets USP, 30 mg - Container Label
Fexofenadine Hydrochloride Tablets USP, 30 mg - Blister Foil
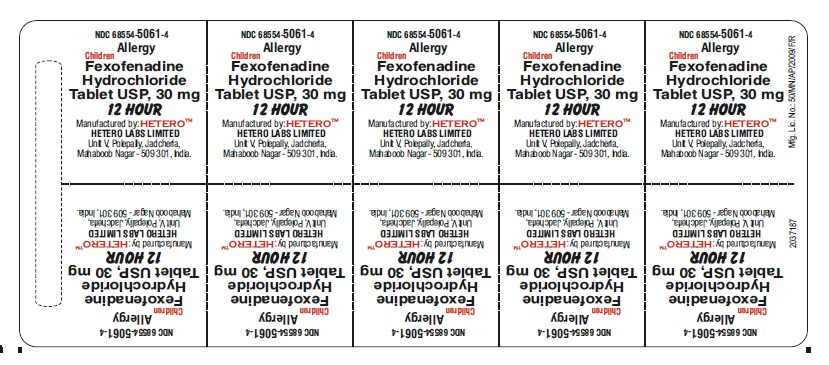
Fexofenadine Hydrochloride Tablets USP, 30 mg - Blister Carton
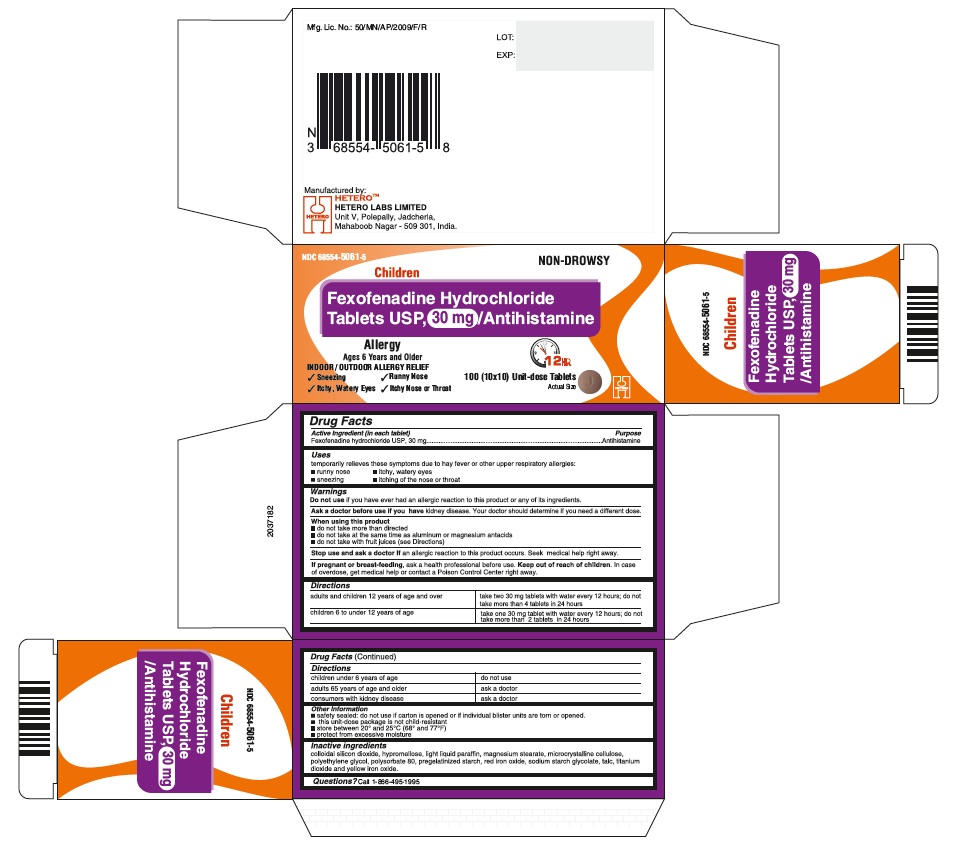
Fexofenadine Hydrochloride Tablets USP, 60 mg - Container Carton
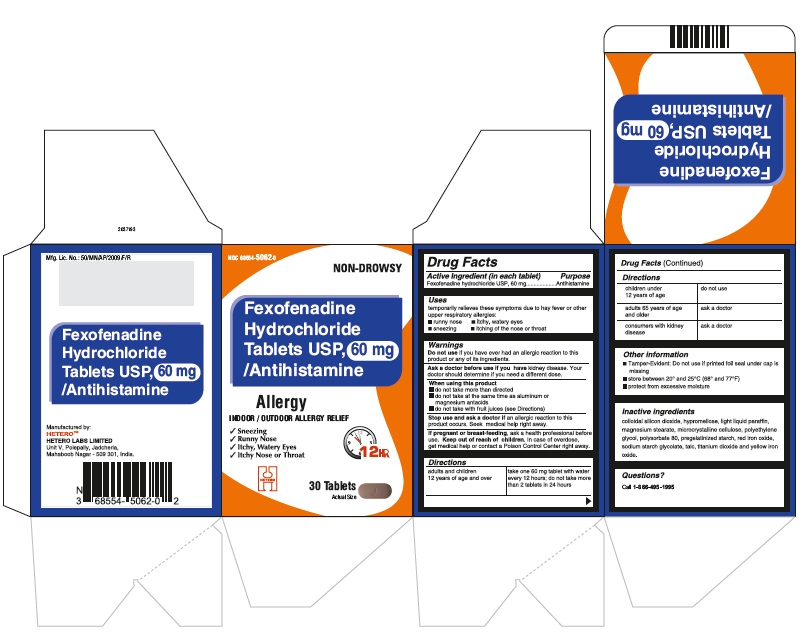
Fexofenadine Hydrochloride Tablets USP, 60 mg - Container Label
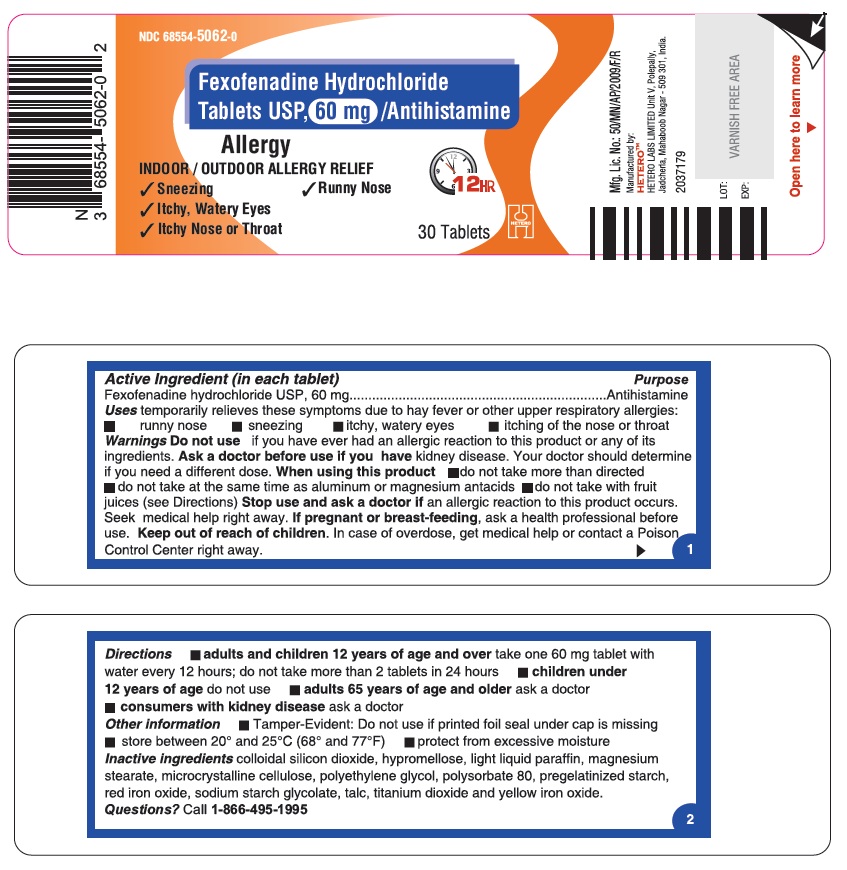
Fexofenadine Hydrochloride Tablets USP, 60 mg - Blister Foil
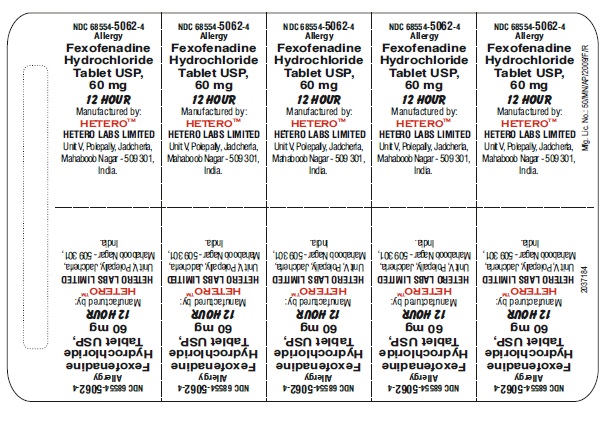
Fexofenadine Hydrochloride Tablets USP, 60 mg - Blister Carton
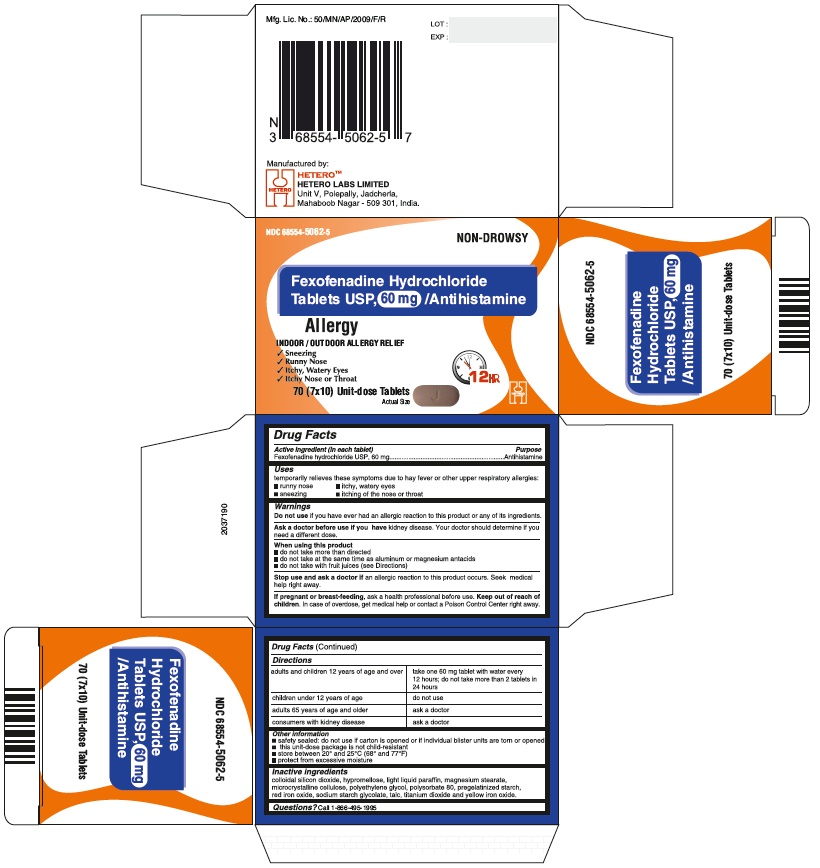
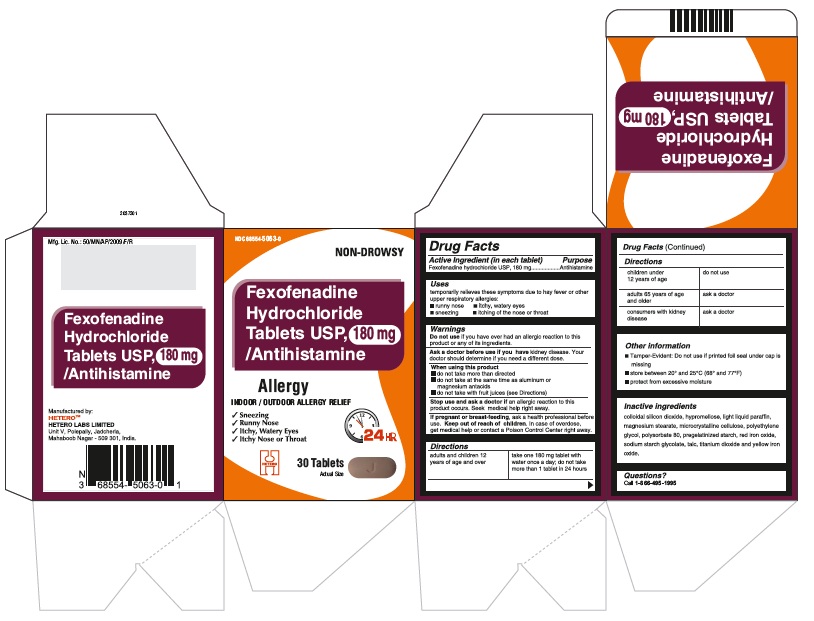
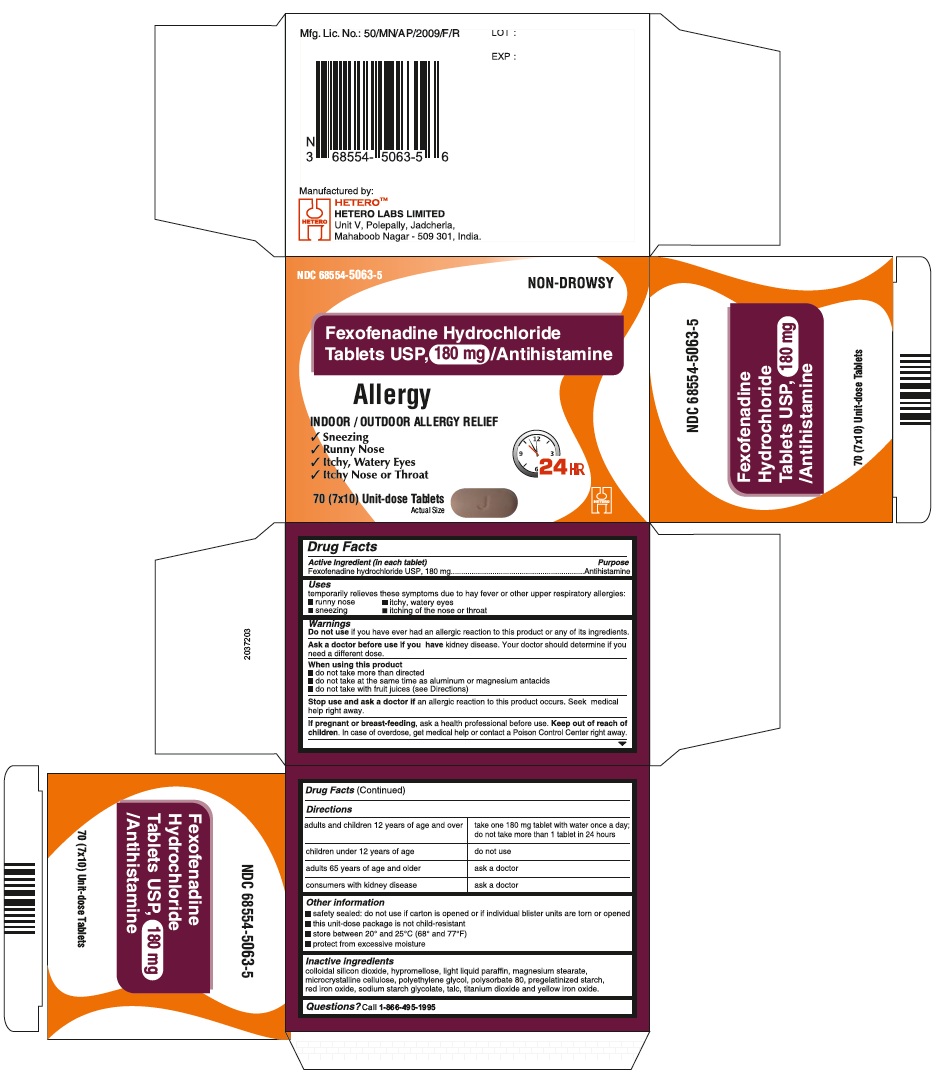
Fexofenadine Hydrochloride Tablets USP, 180 mg - Container Carton
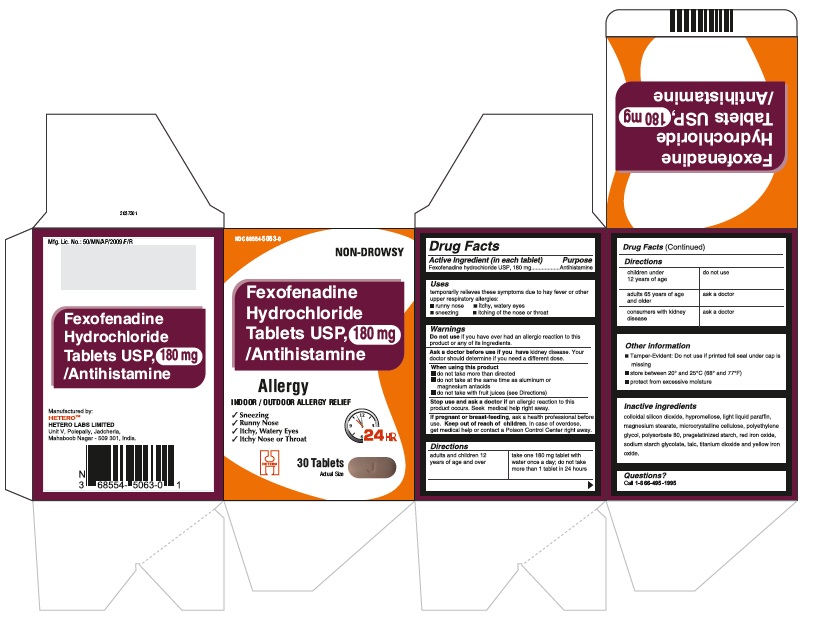
Fexofenadine Hydrochloride Tablets USP, 180 mg - Container Label

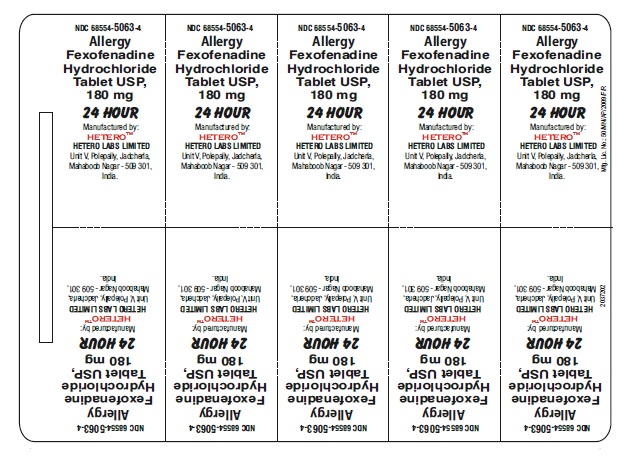
Fexofenadine Hydrochloride Tablets USP, 180 mg - Blister Foil
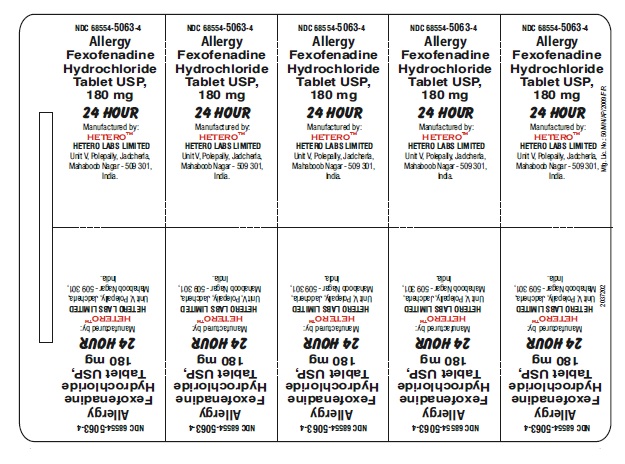
Fexofenadine Hydrochloride Tablets USP, 180 mg - Blister Carton
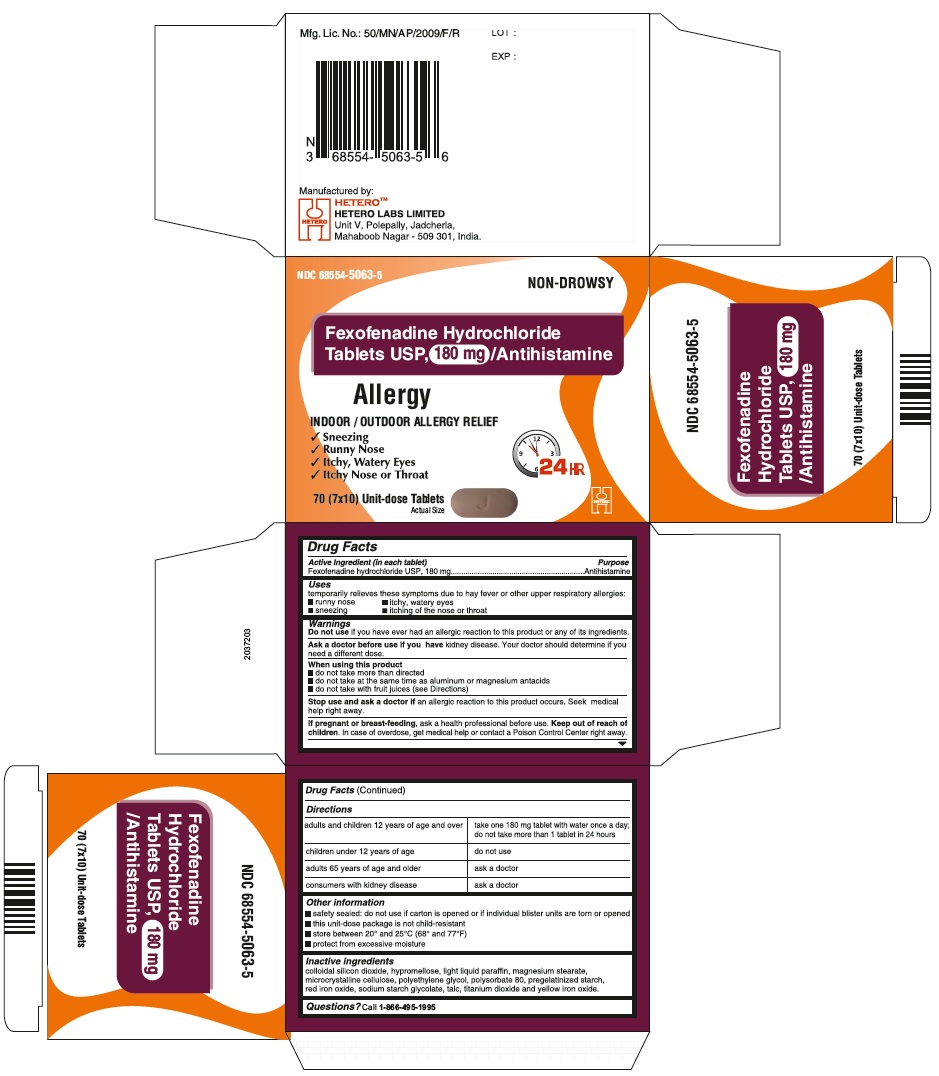
-
INGREDIENTS AND APPEARANCE
FEXOFENADINE HYDROCHLORIDE
fexofenadine hydrochloride tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68554-5061 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 30 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) HYPROMELLOSES (UNII: 3NXW29V3WO) LIGHT MINERAL OIL (UNII: N6K5787QVP) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) POLYSORBATE 80 (UNII: 6OZP39ZG8H) FERRIC OXIDE RED (UNII: 1K09F3G675) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color PINK Score no score Shape ROUND (biconvex) Size 6mm Flavor Imprint Code j;42 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68554-5061-0 1 in 1 CARTON 08/23/2016 1 30 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:68554-5061-1 1 in 1 CARTON 08/23/2016 2 100 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:68554-5061-2 1 in 1 CARTON 08/23/2016 3 500 in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC:68554-5061-5 10 in 1 CARTON 08/23/2016 4 NDC:68554-5061-4 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 5 NDC:68554-5061-6 22 in 1 CARTON 08/23/2016 5 NDC:68554-5061-3 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204097 08/23/2016 FEXOFENADINE HYDROCHLORIDE
fexofenadine hydrochloride tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68554-5062 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 60 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) HYPROMELLOSES (UNII: 3NXW29V3WO) LIGHT MINERAL OIL (UNII: N6K5787QVP) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) POLYSORBATE 80 (UNII: 6OZP39ZG8H) FERRIC OXIDE RED (UNII: 1K09F3G675) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color PINK Score no score Shape OVAL (biconvex) Size 12mm Flavor Imprint Code j;43 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68554-5062-0 1 in 1 CARTON 08/23/2016 1 30 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:68554-5062-1 1 in 1 CARTON 08/23/2016 2 100 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:68554-5062-2 1 in 1 CARTON 08/23/2016 3 500 in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC:68554-5062-5 7 in 1 CARTON 08/23/2016 4 NDC:68554-5062-4 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 5 NDC:68554-5062-6 15 in 1 CARTON 08/23/2016 5 NDC:68554-5062-3 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204097 08/23/2016 FEXOFENADINE HYDROCHLORIDE
fexofenadine hydrochloride tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68554-5063 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 180 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) HYPROMELLOSES (UNII: 3NXW29V3WO) LIGHT MINERAL OIL (UNII: N6K5787QVP) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) POLYSORBATE 80 (UNII: 6OZP39ZG8H) FERRIC OXIDE RED (UNII: 1K09F3G675) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color PINK Score no score Shape CAPSULE (biconvex) Size 18mm Flavor Imprint Code j;44 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68554-5063-0 1 in 1 CARTON 08/23/2016 1 30 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:68554-5063-1 1 in 1 CARTON 08/23/2016 2 100 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:68554-5063-2 1 in 1 CARTON 08/23/2016 3 500 in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC:68554-5063-5 7 in 1 CARTON 08/23/2016 4 NDC:68554-5063-4 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 5 NDC:68554-5063-6 15 in 1 CARTON 08/23/2016 5 NDC:68554-5063-3 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204097 08/23/2016 Labeler - Hetero Labs Limited (917261950)