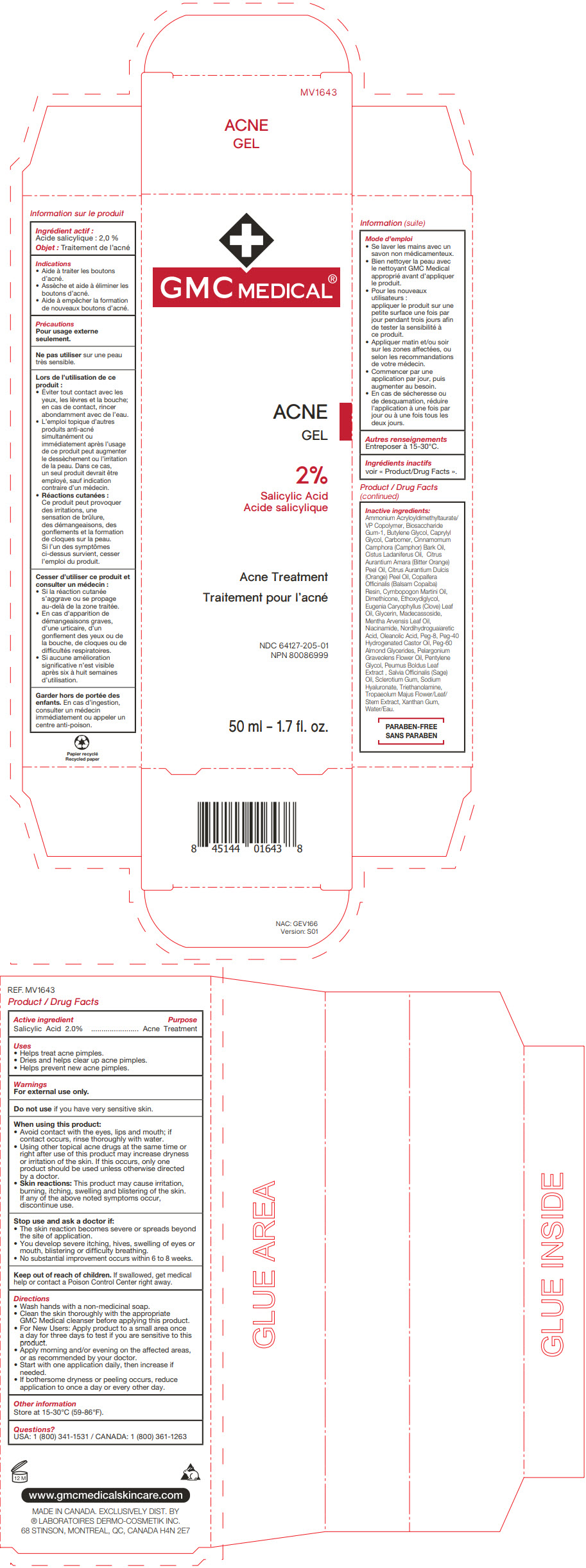
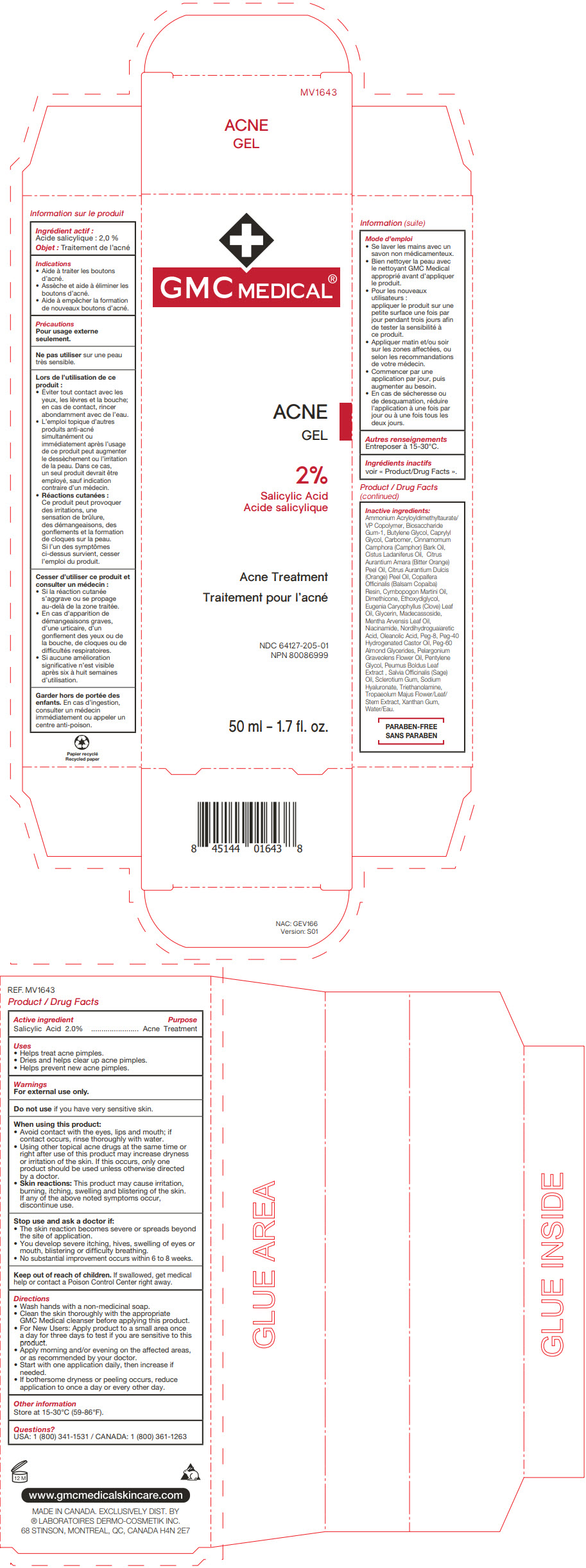
Label: GMC MEDICAL ACNE- salicylic acid gel
- NDC Code(s): 64127-205-01
- Packager: Laboratoires Dermo Cosmetik
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 3, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only.
When using this product
- Avoid contact with the eyes, lips and mouth; if contact occurs, rinse thoroughly with water.
- Using other topical acne drugs at the same time or right after use of this product may increase dryness or irritation of the skin. If this occurs, only one product should be used unless otherwise directed by a doctor.
- Skin reactions:This product may cause irritation, burning, itching, swelling and blistering of the skin. If any of the above noted symptoms occur, discontinue use.
-
Directions
- Wash hands with a non-medicinal soap.
- Clean the skin thoroughly with the appropriate GMC Medical cleanser before applying this product.
- For New Users: Apply product to a small area once a day for three days to test if you are sensitive to this product.
- Apply morning and/or evening on the affected areas, or as recommended by your doctor.
- Start with one application daily, then increase if needed.
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
- Other information
- Questions?
-
Inactive ingredients
Ammonium Acryloyldimethyltaurate/VP Copolymer, Biosaccharide Gum-1, Butylene Glycol, Caprylyl Glycol, Carbomer, Cinnamomum Camphora (Camphor) Bark Oil, Cistus Ladaniferus Oil, Citrus Aurantium Amara (Bitter Orange) Peel Oil, Citrus Aurantium Dulcis (Orange) Peel Oil, Copaifera Officinalis (Balsam Copaiba) Resin, Cymbopogon Martini Oil, Dimethicone, Ethoxydiglycol, Eugenia Caryophyllus (Clove) Leaf Oil, Glycerin, Madecassoside, Mentha Arvensis Leaf Oil, Niacinamide, Nordihydroguaiaretic Acid, Oleanolic Acid, Peg-8, Peg-40 Hydrogenated Castor Oil, Peg-60 Almond Glycerides, Pelargonium Graveolens Flower Oil, Pentylene Glycol, Peumus Boldus Leaf Extract , Salvia Officinalis (Sage) Oil, Sclerotium Gum, Sodium Hyaluronate, Triethanolamine, Tropaeolum Majus Flower/Leaf/Stem Extract, Xanthan Gum, Water/Eau.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 50 ml Tube Carton
-
INGREDIENTS AND APPEARANCE
GMC MEDICAL ACNE
salicylic acid gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64127-205 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) BIOSACCHARIDE GUM-1 (UNII: BB4PU4V09H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) CAMPHOR OIL (UNII: 75IZZ8Y727) LABDANUM OIL (UNII: 67GS9BGA2X) BITTER ORANGE OIL (UNII: 9TLV70SV6I) ORANGE OIL, COLD PRESSED (UNII: AKN3KSD11B) COPAIFERA OFFICINALIS RESIN (UNII: 1VH544O5AT) PALMAROSA OIL (UNII: 0J3G3O53ST) DIMETHICONE (UNII: 92RU3N3Y1O) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) CLOVE LEAF OIL (UNII: VCA5491KVF) GLYCERIN (UNII: PDC6A3C0OX) MADECASSOSIDE (UNII: CQ2F5O6YIY) MENTHA ARVENSIS LEAF OIL (UNII: 1AEY1M553N) NIACINAMIDE (UNII: 25X51I8RD4) OLEANOLIC ACID (UNII: 6SMK8R7TGJ) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) PEG-60 ALMOND GLYCERIDES (UNII: 4Y0E651N0F) PELARGONIUM GRAVEOLENS FLOWER OIL (UNII: 3K0J1S7QGC) PENTYLENE GLYCOL (UNII: 50C1307PZG) SAGE OIL (UNII: U27K0H1H2O) BETASIZOFIRAN (UNII: 2X51AD1X3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) TROLAMINE (UNII: 9O3K93S3TK) XANTHAN GUM (UNII: TTV12P4NEE) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64127-205-01 1 in 1 CARTON 11/26/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 11/26/2018 07/31/2024 Labeler - Laboratoires Dermo Cosmetik (249335480) Establishment Name Address ID/FEI Business Operations Entreprises Importfab Inc. 248586117 manufacture(64127-205)