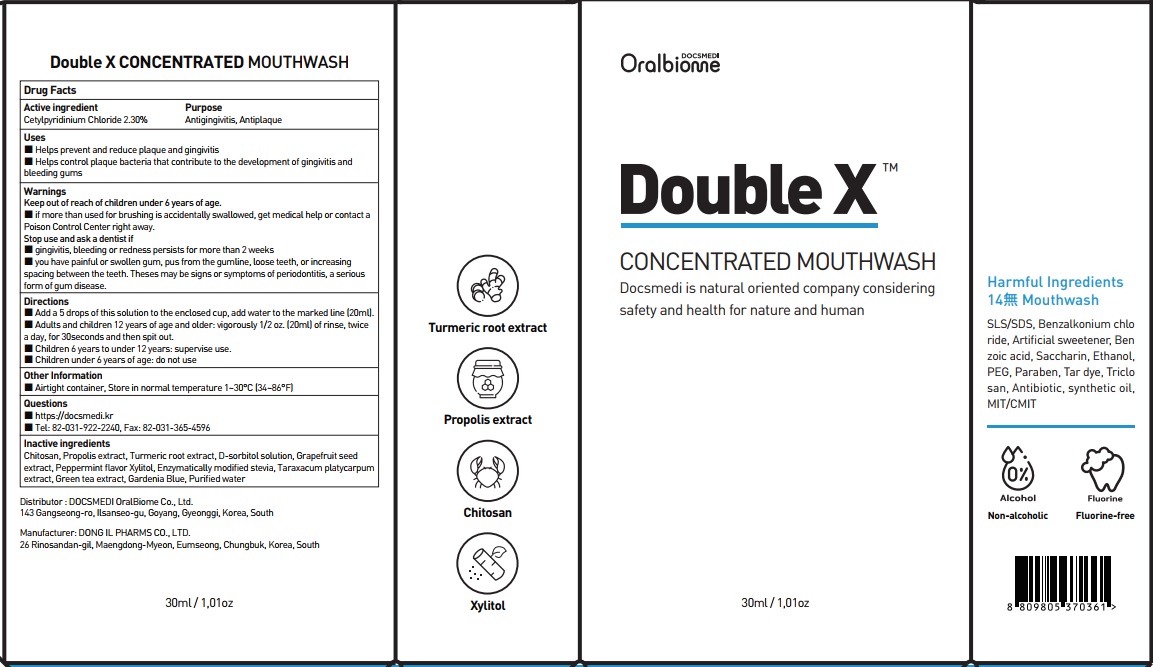
Label: DOUBLE X CONCENTRATED- cetylpyridinium chloride mouthwash
- NDC Code(s): 76670-0012-1, 76670-0012-2
- Packager: DOCSMEDI OralBiome Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENTS
- PURPOSE
-
WARNINGS
Keep out of reach of children under 6 years of age.
■ if more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Stop use and ask a dentist if
■ gingivitis, bleeding or redness persists for more than 2 weeks
■ you have painful or swollen gum, pus from the gumline, loose teeth, or increasing spacing between the teeth. Theses may be signs or symptoms of periodontitis, a serious form of gum disease. - KEEP OUT OF REACH OF CHILDREN
- Uses
-
Directions
■ Add a 5 drops of this solution to the enclosed cup, add water to the marked line (20ml).
■ Adults and children 12 years of age and older: vigorously 1/2 oz. (20ml) of rinse, twice a day, for 30seconds and then spit out.
■ Children 6 years to under 12 years: supervise use.
■ Children under 6 years of age: do not use - Other Information
- Questions
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DOUBLE X CONCENTRATED
cetylpyridinium chloride mouthwashProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76670-0012 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Cetylpyridinium Chloride (UNII: D9OM4SK49P) (CETYLPYRIDINIUM - UNII:CUB7JI0JV3) Cetylpyridinium Chloride 2.30 g in 100 mL Inactive Ingredients Ingredient Name Strength PROPOLIS WAX (UNII: 6Y8XYV2NOF) TURMERIC (UNII: 856YO1Z64F) SORBITOL (UNII: 506T60A25R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76670-0012-2 2 in 1 CARTON 01/02/2022 1 NDC:76670-0012-1 30 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M021 01/02/2022 Labeler - DOCSMEDI OralBiome Co., Ltd. (694505169) Registrant - DOCSMEDI OralBiome Co., Ltd. (694505169) Establishment Name Address ID/FEI Business Operations DONG IL PHARMS CO., LTD. 557810721 manufacture(76670-0012)