Label: ALWAYS LIQUID FOUNDATION MAKEUP SUNSCREEN BROAD SPECTRUM SPF 15 - COCOA- homosalate, octisalate, zinc oxide liquid
- NDC Code(s): 68828-803-01
- Packager: JAFRA COSMETICS INTERNATIONAL
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
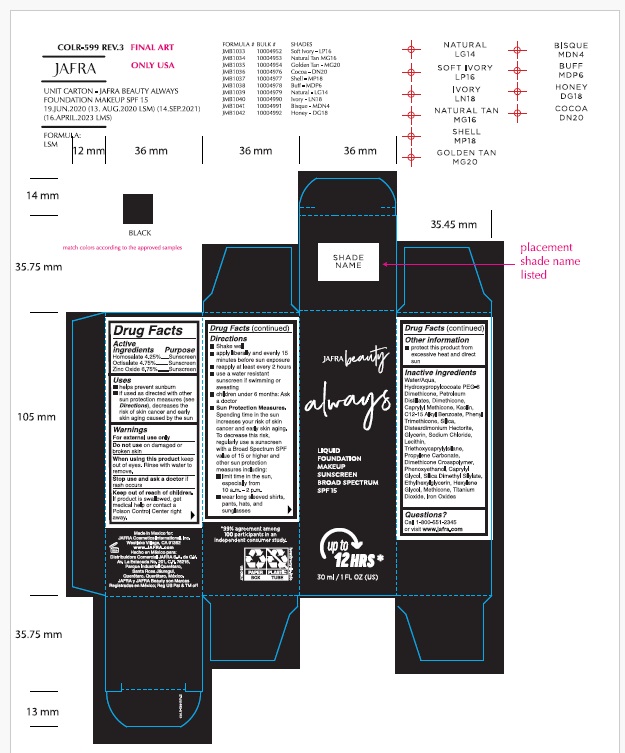
- ACTIVE INGREDIENT
- Purpose
- Uses
- Warnings
- KEEP OUT OF REACH OF CHILDREN
-
Directions
- Shake well
- apply liberally and evenly 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
- children under 6 months: Ask a doctor
- Sun Protection Measures.Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- Other information
-
Inactive Ingredients.
Water/Aqua, Hydroxypropylcocoate PEG-8 Dimethicone, Petroleum Distillates, Dimethicone, Caprylyl Methicone, Kaolin, C12-15 Alkyl Benzoate, Phenyl Trimethicone, Silica, Disteardimonium Hectorite, Glycerin, Sodium Chloride, Lecithin, Triethoxycaprylylsilane, Propylene Carbonate, Dimethicone Crosspolymer, Phenoxyethanol, Caprylyl Glycol, Silica Dimethyl Silylate, Ethylhexylglycerin, Hexylene Glycol, Methicone, Titanium Dioxide, Iron Oxides
- Questions?
- Product label
-
INGREDIENTS AND APPEARANCE
ALWAYS LIQUID FOUNDATION MAKEUP SUNSCREEN BROAD SPECTRUM SPF 15 - COCOA
homosalate, octisalate, zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68828-803 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 4.25 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 4.75 g in 100 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.75 g in 100 mL Inactive Ingredients Ingredient Name Strength SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) SODIUM CHLORIDE (UNII: 451W47IQ8X) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) WATER (UNII: 059QF0KO0R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GLYCERIN (UNII: PDC6A3C0OX) HEXYLENE GLYCOL (UNII: KEH0A3F75J) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) DIMETHICONE (UNII: 92RU3N3Y1O) HYDROXYPROPYLCOCOATE PEG-8 DIMETHICONE (UNII: 8TE0BZU36S) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) METHICONE (20 CST) (UNII: 6777U11MKT) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PHENOXYETHANOL (UNII: HIE492ZZ3T) KAOLIN (UNII: 24H4NWX5CO) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) PROPYLENE CARBONATE (UNII: 8D08K3S51E) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68828-803-01 1 in 1 CARTON 01/18/2024 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/18/2024 Labeler - JAFRA COSMETICS INTERNATIONAL (041676479) Establishment Name Address ID/FEI Business Operations Distribuidora Comercial Jafra, S.A. de C.V. 951612777 manufacture(68828-803)