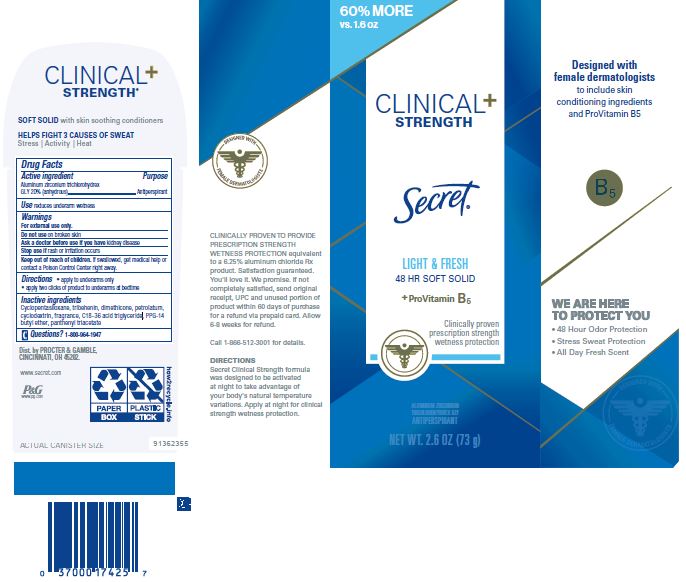
Label: SECRET CLINICAL STRENGTH SOFT LIGHT AND FRESH- aluminum zirconium trichlorohydrex gly cream
-
NDC Code(s):
69423-138-01,
69423-138-02,
69423-138-14,
69423-138-45, view more69423-138-73
- Packager: The Procter & Gamble Manufacturing Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
- Warnings
- Directions
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 73 g Cylinder Carton
-
INGREDIENTS AND APPEARANCE
SECRET CLINICAL STRENGTH SOFT LIGHT AND FRESH
aluminum zirconium trichlorohydrex gly creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69423-138 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM ZIRCONIUM TRICHLOROHYDREX GLY (UNII: T27D6T99LH) (ALUMINUM ZIRCONIUM TRICHLOROHYDREX GLY - UNII:T27D6T99LH) ALUMINUM ZIRCONIUM TRICHLOROHYDREX GLY 20 g in 100 g Inactive Ingredients Ingredient Name Strength C18-36 ACID TRIGLYCERIDE (UNII: ZRA72DR3R7) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) PETROLATUM (UNII: 4T6H12BN9U) TRIBEHENIN (UNII: 8OC9U7TQZ0) PPG-14 BUTYL ETHER (UNII: R199TJT95T) BETADEX (UNII: JV039JZZ3A) PANTHENOL TRIACETATE, (+)- (UNII: 1206E8961B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69423-138-45 1 in 1 CARTON 11/08/2016 01/01/2025 1 45 g in 1 CYLINDER; Type 0: Not a Combination Product 2 NDC:69423-138-73 1 in 1 CARTON 11/08/2016 11/01/2024 2 73 g in 1 CYLINDER; Type 0: Not a Combination Product 3 NDC:69423-138-14 1 in 1 CARTON 11/08/2016 10/01/2020 3 14 g in 1 CYLINDER; Type 0: Not a Combination Product 4 NDC:69423-138-01 2 in 1 CELLO PACK 11/08/2016 12/30/2020 4 1 in 1 CARTON 4 14 g in 1 CYLINDER; Type 0: Not a Combination Product 5 NDC:69423-138-02 3 in 1 BLISTER PACK 01/25/2019 01/01/2025 5 1 in 1 CARTON 5 45 g in 1 CYLINDER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M019 11/08/2016 01/01/2025 Labeler - The Procter & Gamble Manufacturing Company (004238200) Establishment Name Address ID/FEI Business Operations The Procter & Gamble Manufacturing Company 017745779 manufacture(69423-138)