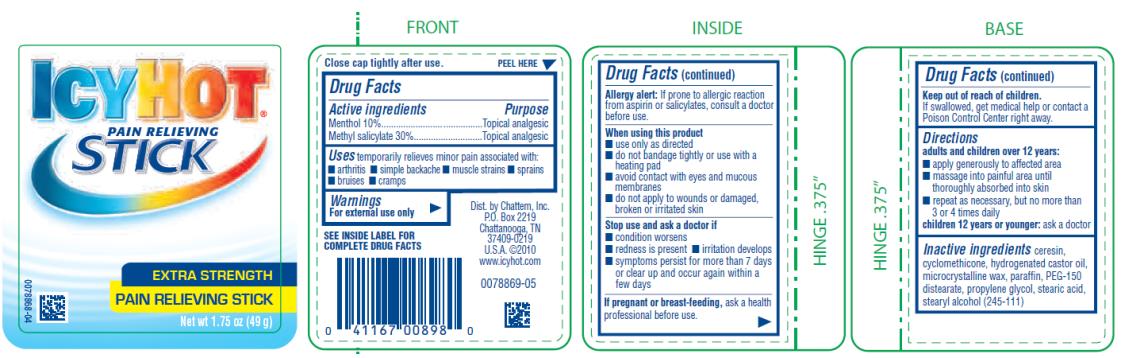
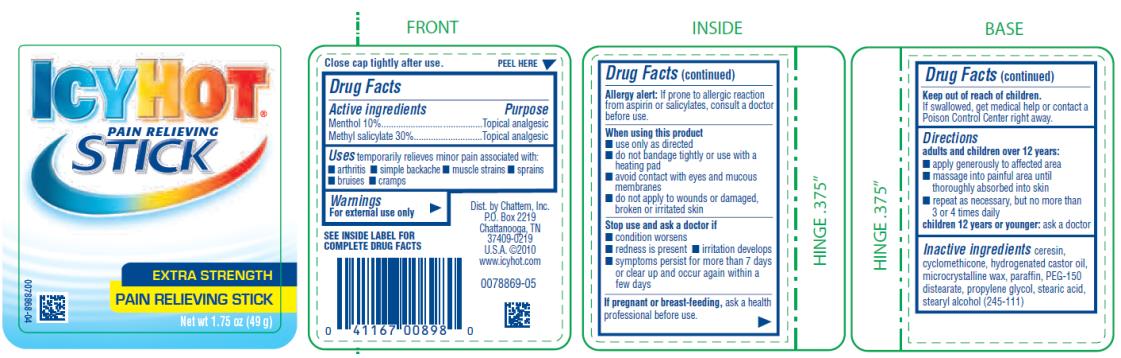
Label: ICY HOT PAIN RELIEVING STICK- menthol and methyl salicylate stick
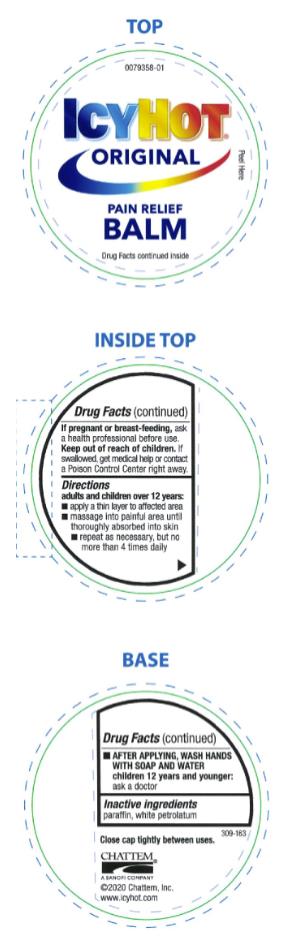
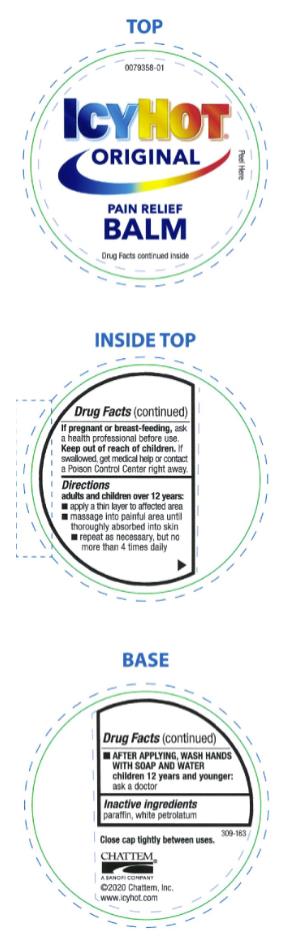
ICY HOT PAIN RELIEVING BALM- menthol and methyl salicylate ointment
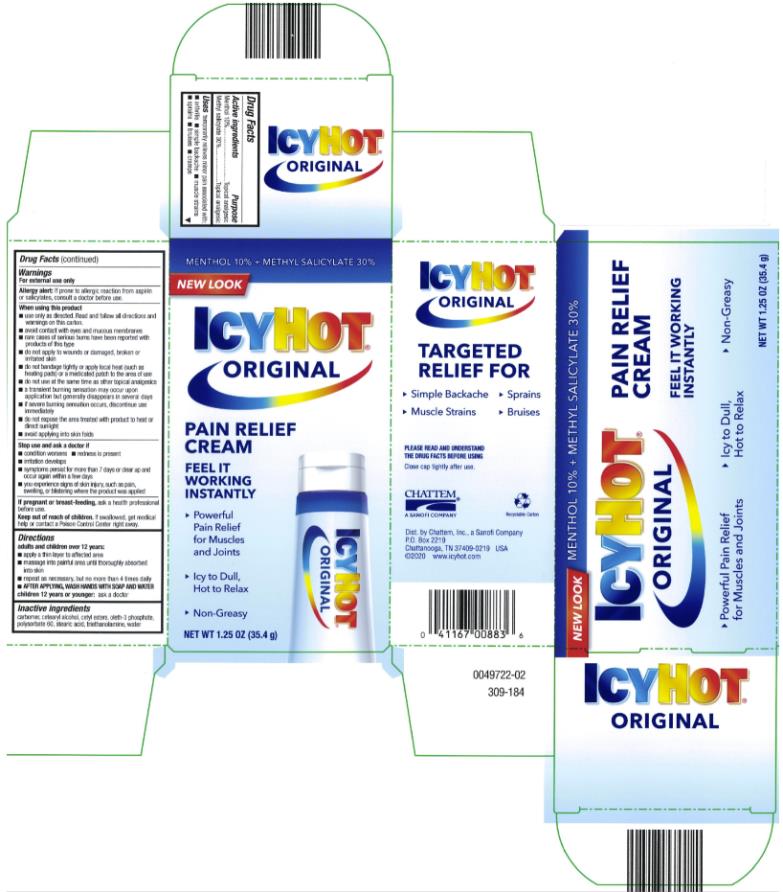
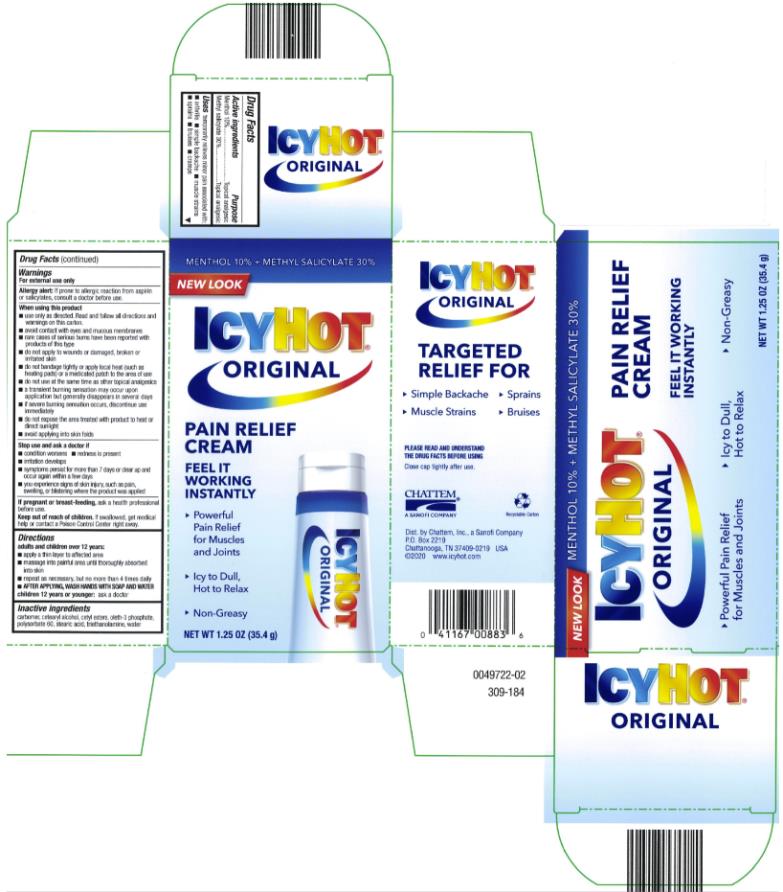
ICY HOT PAIN RELIEVING CREAM- menthol and methyl salicylate cream
-
NDC Code(s):
41167-0087-9,
41167-0088-1,
41167-0088-5,
41167-0088-8, view more41167-0089-8
- Packager: Chattem, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
SEE INSIDE LABEL FOR COMPLETE DRUG FACTS
Allergy Alert: If prone to allergic reaction from aspirin or salicylates, consult a doctor before use.
When using this product
■ use only as directed. Read and follow all directions and warnings on this label.
■ avoid contact with eyes and mucous membranes
■ rare cases of serious burns have been reported with products of this type
■ do not apply to wounds or damaged, broken or irritated skin
■ do not bandage tightly or apply local heat (such as heating pads) or a medicated patch to the area of use
■ a transient burning sensation may occur upon application but generally disappears in several days
■ if severe burning sensation occurs, discontinue use immediately
■ do not expose the area treated with product to heat or direct sunlight
- Directions
- Inactive ingredients
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
Allergy alert: if prone to allergic reaction from aspirin or salicylates, consult a doctor before use
When using this product
■ use only as directed. Read and follow all directions and warnings on this label.
■ avoid contact with eyes and mucous membranes
■ rare cases of serious burns have been reported with products of this type
■ do not apply to wounds or damaged, broken or irritated skin
■ do not bandage tightly or apply local heat (such as heating pads) or a medicated patch to the area of use
■ do not use at the same time as other topical analgesics
■ a transient burning sensation may occur upon application but generally disappears in several days
■ if severe burning sensation occurs, discontinue use immediately
■ do not expose the area treated with product to heat or direct sunlight
■ avoid applying into skin folds
- Directions
- Inactive ingredients
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
Allergy alert: if prone to allergic reaction from aspirin or salicylates, consult a doctor before use
When using this product
■ use only as directed. Read and follow all directions and warnings on this carton.
■ avoid contact with eyes and mucous membranes
■ rare cases of serious burns have been reported with products of this type
■ do not apply to wounds or damaged, broken or irritated skin
■ do not bandage tightly or apply local heat (such as heating pads) or a medicated patch to the area of use
■ do not use at the same time as other topical analgesics
■ a transient burning sensation may occur upon application but generally disappears in several days
■ if severe burning sensation occurs, discontinue use immediately
■ do not expose the area treated with product to heat or direct sunlight
■ avoid applying into skin folds
- Directions
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ICY HOT PAIN RELIEVING STICK
menthol and methyl salicylate stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41167-0089 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 10 g in 100 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 30 g in 100 g Inactive Ingredients Ingredient Name Strength CERESIN (UNII: Q1LS2UJO3A) CYCLOMETHICONE (UNII: NMQ347994Z) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) PARAFFIN (UNII: I9O0E3H2ZE) PEG-150 DISTEARATE (UNII: 6F36Q0I0AC) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) STEARIC ACID (UNII: 4ELV7Z65AP) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41167-0089-8 49 g in 1 JAR; Type 0: Not a Combination Product 06/01/1991 01/31/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 06/01/1991 01/31/2023 ICY HOT PAIN RELIEVING BALM
menthol and methyl salicylate ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41167-0087 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 7.6 g in 100 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 29 g in 100 g Inactive Ingredients Ingredient Name Strength PARAFFIN (UNII: I9O0E3H2ZE) PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41167-0087-9 99 g in 1 JAR; Type 0: Not a Combination Product 06/01/1991 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 06/01/1991 ICY HOT PAIN RELIEVING CREAM
menthol and methyl salicylate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41167-0088 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 10 g in 100 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 30 g in 100 g Inactive Ingredients Ingredient Name Strength CETYL ESTERS WAX (UNII: D072FFP9GU) OLETH-3 PHOSPHATE (UNII: 8Q0Z18J1VL) STEARIC ACID (UNII: 4ELV7Z65AP) WATER (UNII: 059QF0KO0R) TROLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41167-0088-1 1 in 1 CARTON 06/01/1991 1 85 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:41167-0088-5 1 in 1 CARTON 06/01/1991 2 35.4 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:41167-0088-8 1 in 1 CARTON 06/01/1991 07/31/2014 3 44 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 06/01/1991 Labeler - Chattem, Inc. (003336013)