Label: MITCHUM ADVANCE CONTROL- aluminum chlorohydrate aerosol
MITCHUM ADVANCE CONTROL SPORT- aluminum chlorohydrate aerosol
MITCHUM ADVANCE CONTROL POWDER FRESH- aluminum chlorohydrate aerosol
MITCHUM ADVANCE CONTROL PURE FRESH- aluminum chlorohydrate aerosol
-
NDC Code(s):
10967-635-04,
10967-636-04,
10967-637-04,
10967-638-04, view more10967-649-04
- Packager: Revlon Consumer Products Corp
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 7, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- INSTRUCTIONS FOR USE
-
WARNINGS
Warnings:
Do not use on broken skin
Stop Use if rash or irritation occurs.
When using this product, keep away from face and mouth to avoid breathing in.
Avoid spraying in eyes
Contents under pressure
Do not place in hot water or near radiators, stoves, or other sources of heat.
Do not puncture or incinerate container or store at temperatures over 50C
Flammable Do not use in presence of flame or spark.
Keep out of reach of children
- QUESTIONS
- Uses
- Purpose
-
Inactive Ingredients
Hydrofluorocarbon 152a,
Cyclopentasiloxane, Butane, C12-15 Alkyl Benzoate,
Isopropyl Myristate, Disteardimonium Hectorite,
Propylene Carbonate, Tocopheryl Acetate, Aloe
Barbadensis Leaf Extract, Parfum (Fragrance), Linalool,
Benzyl Salicylate, Citronellol, Hydroxycitronellal, Hexyl
Cinnamal, Limonene, Benzyl Benzoate, Geraniol,
Cinnamyl Alcohol, Alpha-Isomethyl Ionone. 24309 - DOSAGE & ADMINISTRATION
- Keep out of Reach of Children
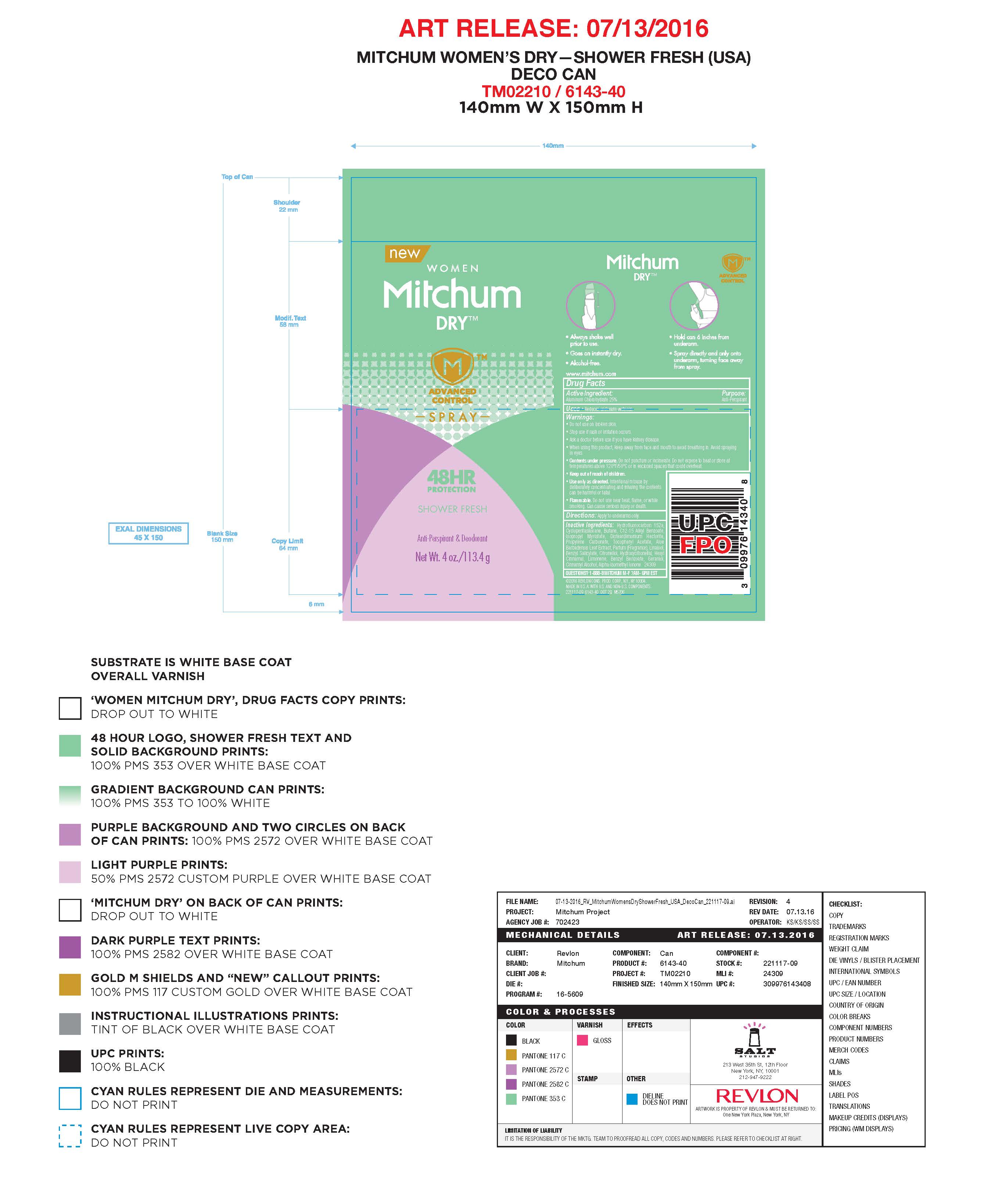
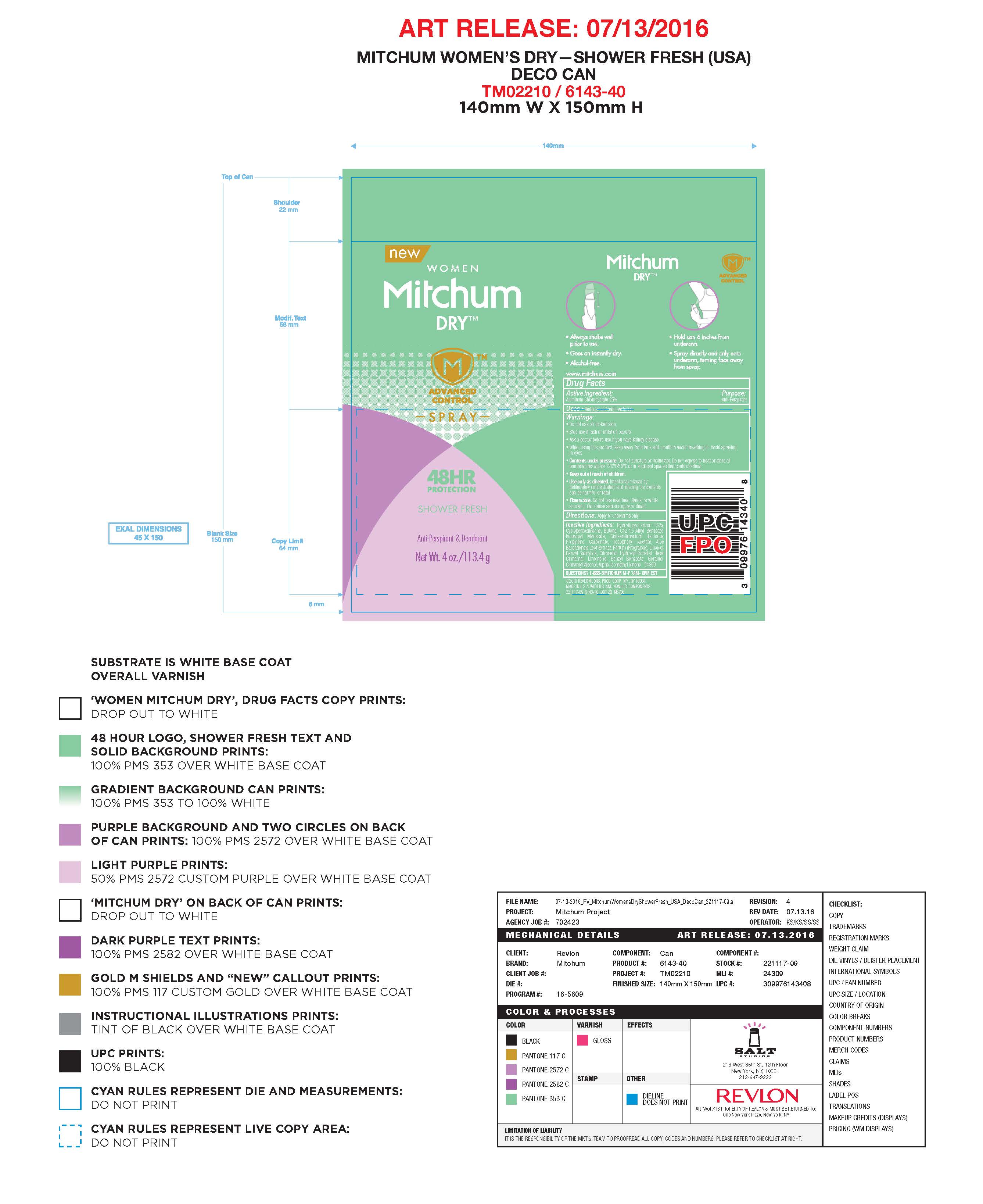
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MITCHUM ADVANCE CONTROL
aluminum chlorohydrate aerosolProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10967-635 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 0.25 g in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ALOE VERA LEAF (UNII: ZY81Z83H0X) PROPYLENE CARBONATE (UNII: 8D08K3S51E) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) GERANIOL (UNII: L837108USY) COUMARIN (UNII: A4VZ22K1WT) LIMONENE, (+)- (UNII: GFD7C86Q1W) 1,1-DIFLUOROETHANE (UNII: 0B1U8K2ME0) BUTANE (UNII: 6LV4FOR43R) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) LINALOOL, (+/-)- (UNII: D81QY6I88E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10967-635-04 113.4 mL in 1 CANISTER; Type 0: Not a Combination Product 12/07/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part350 12/07/2015 MITCHUM ADVANCE CONTROL SPORT
aluminum chlorohydrate aerosolProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10967-636 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 0.25 g in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ALOE VERA LEAF (UNII: ZY81Z83H0X) PROPYLENE CARBONATE (UNII: 8D08K3S51E) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) GERANIOL (UNII: L837108USY) COUMARIN (UNII: A4VZ22K1WT) LIMONENE, (+)- (UNII: GFD7C86Q1W) 1,1-DIFLUOROETHANE (UNII: 0B1U8K2ME0) BUTANE (UNII: 6LV4FOR43R) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) LINALOOL, (+/-)- (UNII: D81QY6I88E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10967-636-04 113.4 mL in 1 CANISTER; Type 0: Not a Combination Product 12/07/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part350 12/07/2015 MITCHUM ADVANCE CONTROL
aluminum chlorohydrate aerosolProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10967-638 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 0.25 g in 1 mL Inactive Ingredients Ingredient Name Strength ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) 1,1-DIFLUOROETHANE (UNII: 0B1U8K2ME0) BUTANE (UNII: 6LV4FOR43R) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ALOE VERA LEAF (UNII: ZY81Z83H0X) PROPYLENE CARBONATE (UNII: 8D08K3S51E) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) COUMARIN (UNII: A4VZ22K1WT) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10967-638-04 113.4 mL in 1 CANISTER; Type 0: Not a Combination Product 12/07/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part350 12/07/2015 MITCHUM ADVANCE CONTROL POWDER FRESH
aluminum chlorohydrate aerosolProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10967-637 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 0.25 g in 1 mL Inactive Ingredients Ingredient Name Strength LIMONENE, (+)- (UNII: GFD7C86Q1W) 1,1-DIFLUOROETHANE (UNII: 0B1U8K2ME0) BUTANE (UNII: 6LV4FOR43R) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) LINALOOL, (+/-)- (UNII: D81QY6I88E) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ALOE VERA LEAF (UNII: ZY81Z83H0X) PROPYLENE CARBONATE (UNII: 8D08K3S51E) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) GERANIOL (UNII: L837108USY) COUMARIN (UNII: A4VZ22K1WT) BENZYL SALICYLATE (UNII: WAO5MNK9TU) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10967-637-04 113.4 mL in 1 CANISTER; Type 0: Not a Combination Product 12/07/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part350 12/07/2015 MITCHUM ADVANCE CONTROL PURE FRESH
aluminum chlorohydrate aerosolProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10967-649 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 0.25 g in 1 mL Inactive Ingredients Ingredient Name Strength LIMONENE, (+)- (UNII: GFD7C86Q1W) 1,1-DIFLUOROETHANE (UNII: 0B1U8K2ME0) BUTANE (UNII: 6LV4FOR43R) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) LINALOOL, (+/-)- (UNII: D81QY6I88E) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ALOE VERA LEAF (UNII: ZY81Z83H0X) PROPYLENE CARBONATE (UNII: 8D08K3S51E) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) GERANIOL (UNII: L837108USY) COUMARIN (UNII: A4VZ22K1WT) BENZYL SALICYLATE (UNII: WAO5MNK9TU) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10967-649-04 113.4 mL in 1 CANISTER; Type 0: Not a Combination Product 12/07/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part350 12/07/2015 Labeler - Revlon Consumer Products Corp (788820165) Establishment Name Address ID/FEI Business Operations American Spraytech 137135237 manufacture(10967-635, 10967-636, 10967-637, 10967-638, 10967-649)