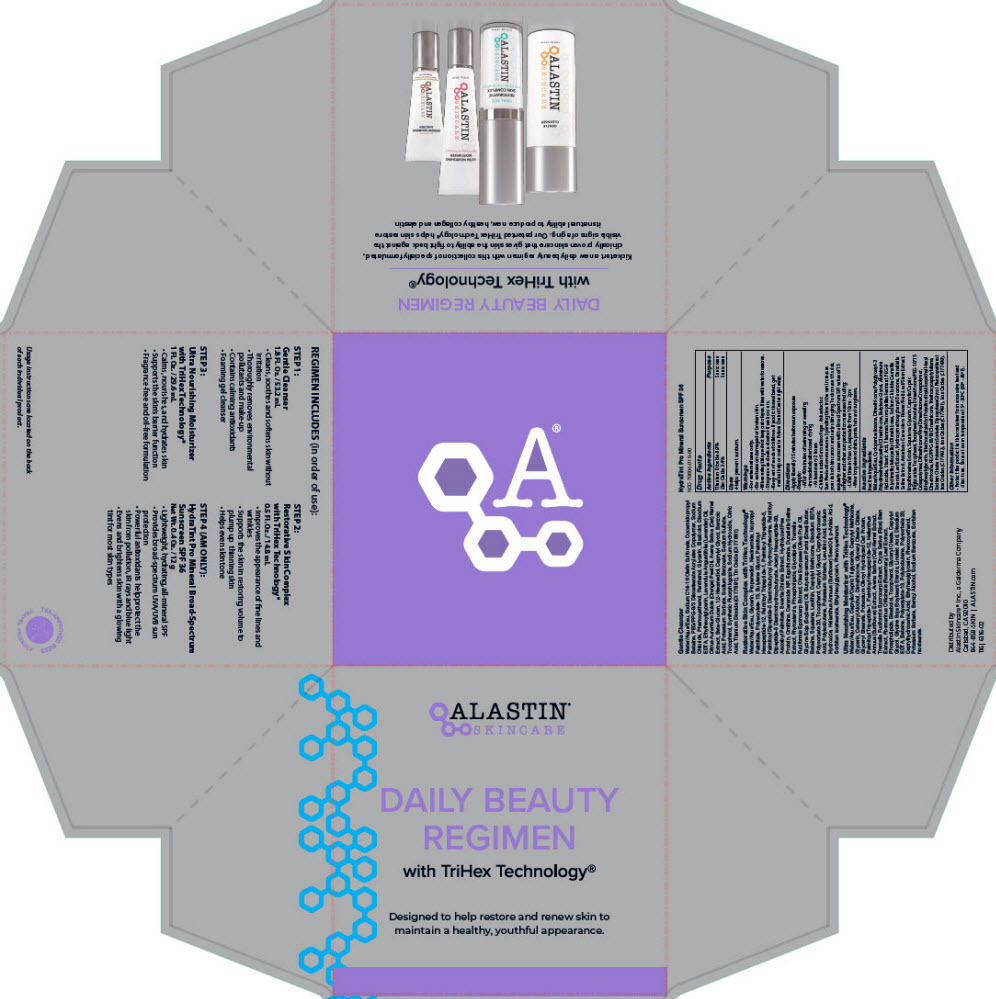
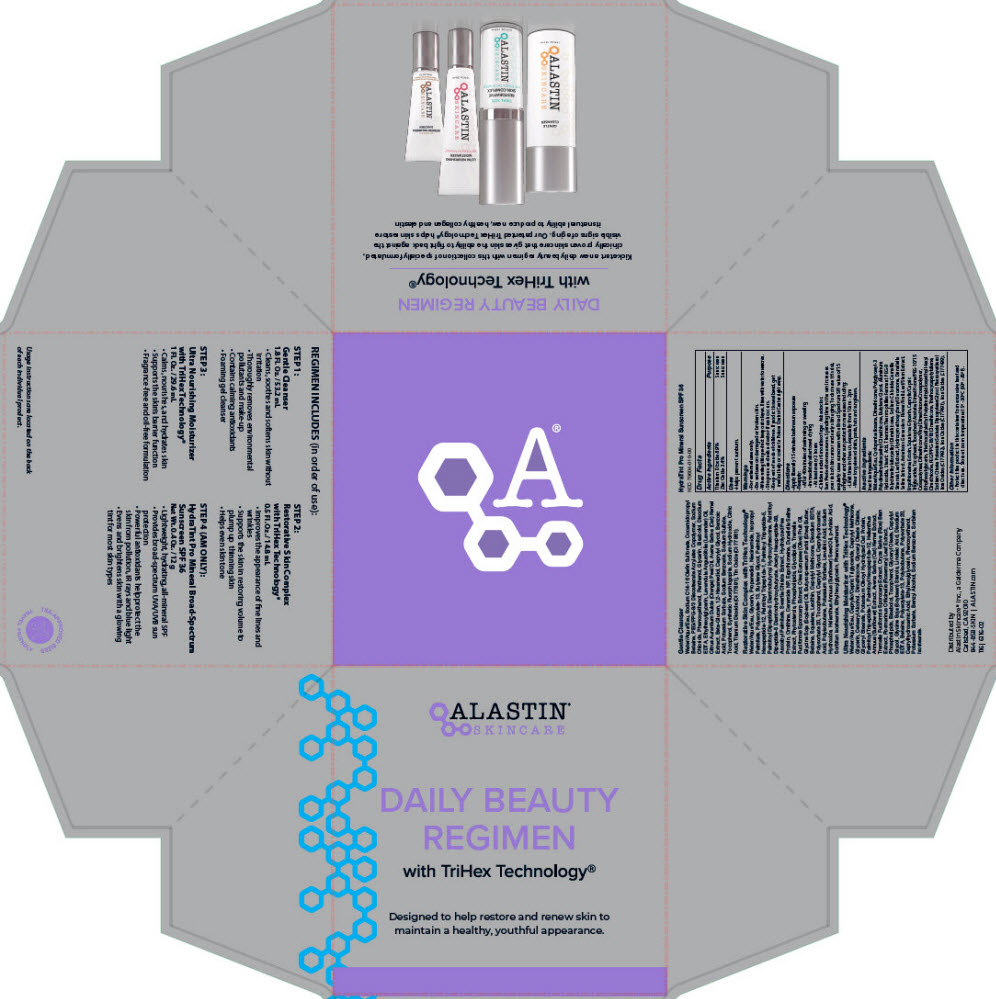
Label: DAILY BEAUTY REGIMEN WITH TRIHEX TECHNOLOGY- titanium dioxide and zinc oxide kit
- NDC Code(s): 70604-011-00, 70604-050-00
- Packager: Alastin Skincare, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure
- Reapply:
- After 40 minutes of swimming or sweating
- Immediately after towel drying
- At least every 2 hours
- Children under 6 months of age: Ask a doctor.
Sun Protection measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:- Limit time in the sun, especially from 10am - 2pm
- Wear long-sleeved shirts, pants, hats and sunglasses.
-
Inactive Ingredients
Inactive Ingredients:
Water/Aqua/Eau, Cyclopentasiloxane, Dimethicone, Polyglyceryl-3 Polydimethylsiloxyethyl Dimethicone, Butylene Glycol, Aluminum Hydroxide, Stearic Acid, Thermus Thermophillus Ferment, PEG-9 Polydimethylsiloxyethyl Dimethicone, Sodium Chloride, Camellia Sinensis Leaf Extract, Hydroxymethoxyphenyl Decanone, Dunaliella Salina Extract, Asteriscus Graveolens Flower/Fruit/Leaf/Stem Extract, Ergothioneine, Ectoin, Squalane, Glycerin, Caprylic/Capric Triglyceride, Tocopherol, Tocopheryl Acetate, Dimethicone/PEG-10/15 Crosspolymer, Dimethicone/Vinyl Dimethicone Crosspolymer, Ethylhexylglycerin, Triethoxysilylethyl Polydimethylsiloxyethyl Hexyl Dimethicone, PEG/PPG-18/18 Dimethicone, Triethoxycaprylylsilane, Sodium Citrate, Potassium Sorbate, Dipropylene Glycol, Phenoxyethanol, Iron Oxides (CI 77492), Iron Oxides (CI 77491), Iron Oxides (CI 77499).
- Other Information
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - Kit Carton
-
INGREDIENTS AND APPEARANCE
DAILY BEAUTY REGIMEN WITH TRIHEX TECHNOLOGY
titanium dioxide and zinc oxide kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70604-050 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70604-050-00 1 in 1 CARTON 09/24/2021 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 12 g Part 2 1 BOTTLE, PLASTIC 53.2 mL Part 3 1 BOTTLE, PUMP 14.8 mL Part 4 1 TUBE 29.6 mL Part 1 of 4 HYDRATINT PRO MINERAL BROAD SPECTRUM SUNSCREEN SPF
titanium dioxide and zinc oxide creamProduct Information Item Code (Source) NDC:70604-011 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.089 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.034 g in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) POLYGLYCERYL-3 POLYDIMETHYLSILOXYETHYL DIMETHICONE (4000 MPA.S) (UNII: RLA2U05Z4Q) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) THERMUS THERMOPHILUS LYSATE (UNII: 775R692494) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) SODIUM CHLORIDE (UNII: 451W47IQ8X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) 1-(4-HYDROXY-3-METHOXYPHENYL)-DECAN-3-ONE (UNII: BO24ID7E9U) DUNALIELLA SALINA (UNII: F4O1DKI9A6) ERGOTHIONEINE (UNII: BDZ3DQM98W) ECTOINE (UNII: 7GXZ3858RY) SQUALANE (UNII: GW89575KF9) GLYCERIN (UNII: PDC6A3C0OX) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) TOCOPHEROL (UNII: R0ZB2556P8) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) DIMETHICONE/PEG-10/15 CROSSPOLYMER (UNII: 21AS8B1BSS) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) TRIETHOXYSILYLETHYL POLYDIMETHYLSILOXYETHYL HEXYL DIMETHICONE (UNII: X75PL53TZJ) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) DIPROPYLENE GLYCOL (UNII: E107L85C40) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Product Characteristics Color BROWN Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70604-011-00 12 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 09/24/2021 Part 2 of 4 GENTLE CLEANSER
cleansing (cold creams, cleansing lotions, liquids, and pads) [skin care preparations (creams, lotions, powder, and sprays)] liquidProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR SODIUM C14-16 OLEFIN SULFONATE (UNII: O9W3D3YF5U) INGR COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) INGR OAT (UNII: Z6J799EAJK) INGR ODETIGLUCAN (UNII: Q6X9CNN54X) INGR PANTHENOL (UNII: WV9CM0O67Z) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR LEVOMENOL (UNII: 24WE03BX2T) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR SODIUM CHLORIDE (UNII: 451W47IQ8X) INGR PANTOLACTONE (UNII: J288D7O0JS) INGR 1,2-HEXANEDIOL (UNII: TR046Y3K1G) INGR CAPRYLYL GLYCOL (UNII: 00YIU5438U) INGR SODIUM BENZOATE (UNII: OJ245FE5EU) INGR POTASSIUM SORBATE (UNII: 1VPU26JZZ4) INGR SODIUM HYDROXIDE (UNII: 55X04QC32I) INGR ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) INGR EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) INGR MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) INGR LAVENDER OIL (UNII: ZBP1YXW0H8) INGR ORANGE OIL, COLD PRESSED (UNII: AKN3KSD11B) INGR STANNIC OXIDE (UNII: KM7N50LOS6) INGR TOCOPHEROL (UNII: R0ZB2556P8) INGR TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 53.2 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic Part 3 of 4 RESTORATIVE SKIN COMPLEX
moisturizing [skin care preparations (creams, lotions, powder, and sprays)] lotionProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR PROPANEDIOL (UNII: 5965N8W85T) INGR NIACINAMIDE (UNII: 25X51I8RD4) INGR ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) INGR POLYACRYLATE-13 (UNII: FS2D4T67EA) INGR BUTYLENE GLYCOL (UNII: 3XUS85K0RA) INGR PALMITOYL HEXAPEPTIDE-12 (UNII: HO4ZT5S86C) INGR PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) INGR PALMITOYL TRIPEPTIDE-5 TRIFLATE (UNII: 4IV5XO4TGK) INGR PALMITOYLLYSYLVALYLDIAMINOBUTYROYLTHREONINE TRIFLUOROACETATE (UNII: CL9S2UKN56) INGR PALMITOYLLYSYLVALYLDIAMINOBUTYRIC ACID TRIFLUOROACETATE (UNII: 79390Q4BYV) INGR ACETYL HEXAPEPTIDE-38 (UNII: XUJ27AR5LA) INGR ASCORBYL PALMITATE (UNII: QN83US2B0N) INGR SWERTIA CHIRAYITA WHOLE (UNII: 98BFC3JT53) INGR PEA PROTEIN (UNII: 7Q50F46595) INGR ORNITHINE (UNII: E524N2IXA3) INGR CERAMIDE NP (UNII: 4370DF050B) INGR ERGOTHIONEINE (UNII: BDZ3DQM98W) INGR DUNALIELLA SALINA (UNII: F4O1DKI9A6) INGR LECITHIN, SOYBEAN (UNII: 1DI56QDM62) INGR TREMELLA FUCIFORMIS FRUITING BODY (UNII: GG8N28393G) INGR OLIVE OIL (UNII: 6UYK2W1W1E) INGR SOYBEAN OIL (UNII: 241ATL177A) INGR SHEA BUTTER (UNII: K49155WL9Y) INGR BETAINE (UNII: 3SCV180C9W) INGR SQUALANE (UNII: GW89575KF9) INGR CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) INGR EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) INGR POLYSORBATE 20 (UNII: 7T1F30V5YH) INGR TOCOPHEROL (UNII: R0ZB2556P8) INGR CAPRYLYL GLYCOL (UNII: 00YIU5438U) INGR CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) INGR POLYISOBUTYLENE (1000 MW) (UNII: 5XB3A63Y52) INGR POTASSIUM SORBATE (UNII: 1VPU26JZZ4) INGR LEVULINIC ACID (UNII: RYX5QG61EI) INGR SODIUM HYDROXIDE (UNII: 55X04QC32I) INGR SUNFLOWER OIL (UNII: 3W1JG795YI) INGR P-ANISIC ACID (UNII: 4SB6Y7DMM3) INGR SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 14.8 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic Part 4 of 4 ULTRA NOURISHING MOISTURIZER
moisturizing [skin care preparations (creams, lotions, powder, and sprays)] lotionProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) INGR CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) INGR DIMETHICONE (UNII: 92RU3N3Y1O) INGR GLYCERYL MONOOLEATE CITRATE (UNII: NLE5KIG74K) INGR GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) INGR PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) INGR PALMITOYL HEXAPEPTIDE-12 (UNII: HO4ZT5S86C) INGR SUNFLOWER OIL (UNII: 3W1JG795YI) INGR OAT (UNII: Z6J799EAJK) INGR TREMELLA FUCIFORMIS FRUITING BODY (UNII: GG8N28393G) INGR RICE BRAN (UNII: R60QEP13IC) INGR ROSEMARY (UNII: IJ67X351P9) INGR LECITHIN, SOYBEAN (UNII: 1DI56QDM62) INGR LEVOMENOL (UNII: 24WE03BX2T) INGR .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) INGR GLYCERYL MONOOLEATE (UNII: C4YAD5F5G6) INGR CAPRYLYL GLYCOL (UNII: 00YIU5438U) INGR SOY STEROL (UNII: PL360EPO9J) INGR LINOLEIC ACID (UNII: 9KJL21T0QJ) INGR EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) INGR BETAINE (UNII: 3SCV180C9W) INGR POLYACRYLATE-13 (UNII: FS2D4T67EA) INGR POLYISOBUTYLENE (1000 MW) (UNII: 5XB3A63Y52) INGR POLYSORBATE 20 (UNII: 7T1F30V5YH) INGR CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR POTASSIUM SORBATE (UNII: 1VPU26JZZ4) INGR BENZYL ALCOHOL (UNII: LKG8494WBH) INGR SODIUM BENZOATE (UNII: OJ245FE5EU) INGR SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 29.6 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 09/24/2021 Labeler - Alastin Skincare, Inc. (085997348)