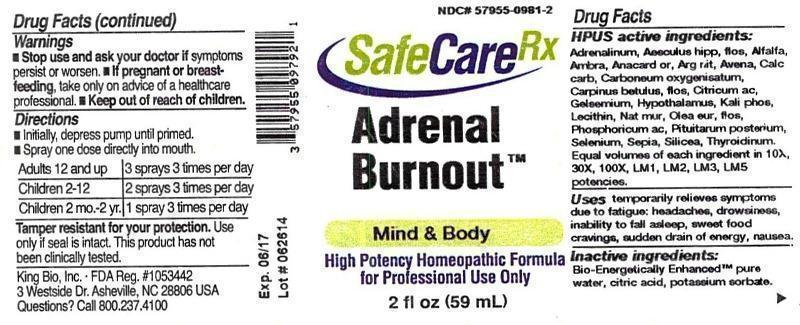
Label: ADRENAL BURNOUT- adrenalinum, aesculus hippocastanum, flos, alfalfa, ambra grisea, anacardium orientale, argentum nitricum, avena sativa, calcarea carbonica, carboneum oxygenisatum, carpinus betulus, flos, citricum acidum, gelsemium sempervirens, hypothalamus, kali phosphoricum, lecithin, natrum muriaticum, olea europaea, flos, phosphoricum acidum, pituitarum posterium, selenium metallicum, sepia, silicea, thyroidinum liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 57955-0981-2 - Packager: King Bio Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 10, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
-
ACTIVE INGREDIENT
HPUS active ingredients: Adrenalinum, Aesculus hippocastanum, flos, Alfalfa, Ambra grisea, Anacardium orientale, Argentum nitricum, Avena sativa, Calcarea carbonica, Carboneum oxygenisatum, Carpinus betulus, flos, Citricum acidum, Gelsemium sempervirens, Hypothalamus, Kali phosphoricum, Lecithin, Natrum muriaticum, Olea europaea, flos, Phosphoricum acidum, Pituitarum posterium, Selenium metallicum, Sepia, Silicea, Thyroidinum. Equal volumes of each ingredient in 10X, 30X, 100X, LM1, LM2, LM3, LM5 potencies.
- PURPOSE
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ADRENAL BURNOUT
adrenalinum, aesculus hippocastanum, flos, alfalfa, ambra grisea, anacardium orientale, argentum nitricum, avena sativa, calcarea carbonica, carboneum oxygenisatum, carpinus betulus, flos, citricum acidum, gelsemium sempervirens, hypothalamus, kali phosphoricum, lecithin, natrum muriaticum, olea europaea, flos, phosphoricum acidum, pituitarum posterium, selenium metallicum, sepia, silicea, thyroidinum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57955-0981 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINEPHRINE (UNII: YKH834O4BH) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 10 [hp_X] in 59 mL AESCULUS HIPPOCASTANUM FLOWER (UNII: KK0Z92II8M) (AESCULUS HIPPOCASTANUM FLOWER - UNII:KK0Z92II8M) AESCULUS HIPPOCASTANUM FLOWER 10 [hp_X] in 59 mL ALFALFA (UNII: DJO934BRBD) (ALFALFA - UNII:DJO934BRBD) ALFALFA 10 [hp_X] in 59 mL AMBERGRIS (UNII: XTC0D02P6C) (AMBERGRIS - UNII:XTC0D02P6C) AMBERGRIS 10 [hp_X] in 59 mL SEMECARPUS ANACARDIUM JUICE (UNII: Y0F0BU8RDU) (SEMECARPUS ANACARDIUM JUICE - UNII:Y0F0BU8RDU) SEMECARPUS ANACARDIUM JUICE 10 [hp_X] in 59 mL SILVER NITRATE (UNII: 95IT3W8JZE) (SILVER CATION - UNII:57N7B0K90A) SILVER NITRATE 10 [hp_X] in 59 mL AVENA SATIVA FLOWERING TOP (UNII: MA9CQJ3F7F) (AVENA SATIVA FLOWERING TOP - UNII:MA9CQJ3F7F) AVENA SATIVA FLOWERING TOP 10 [hp_X] in 59 mL OYSTER SHELL CALCIUM CARBONATE, CRUDE (UNII: 2E32821G6I) (OYSTER SHELL CALCIUM CARBONATE, CRUDE - UNII:2E32821G6I) OYSTER SHELL CALCIUM CARBONATE, CRUDE 10 [hp_X] in 59 mL CARBON MONOXIDE (UNII: 7U1EE4V452) (CARBON MONOXIDE - UNII:7U1EE4V452) CARBON MONOXIDE 10 [hp_X] in 59 mL CARPINUS BETULUS FLOWERING TOP (UNII: QOI241B01F) (CARPINUS BETULUS FLOWERING TOP - UNII:QOI241B01F) CARPINUS BETULUS FLOWERING TOP 10 [hp_X] in 59 mL ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) ANHYDROUS CITRIC ACID 10 [hp_X] in 59 mL GELSEMIUM SEMPERVIRENS ROOT (UNII: 639KR60Q1Q) (GELSEMIUM SEMPERVIRENS ROOT - UNII:639KR60Q1Q) GELSEMIUM SEMPERVIRENS ROOT 10 [hp_X] in 59 mL BOS TAURUS HYPOTHALAMUS (UNII: S6G2NLH4Y7) (BOS TAURUS HYPOTHALAMUS - UNII:S6G2NLH4Y7) BOS TAURUS HYPOTHALAMUS 10 [hp_X] in 59 mL POTASSIUM PHOSPHATE, DIBASIC (UNII: CI71S98N1Z) (PHOSPHATE ION - UNII:NK08V8K8HR) POTASSIUM PHOSPHATE, DIBASIC 10 [hp_X] in 59 mL EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) (EGG PHOSPHOLIPIDS - UNII:1Z74184RGV) EGG PHOSPHOLIPIDS 10 [hp_X] in 59 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 10 [hp_X] in 59 mL OLEA EUROPAEA FLOWER (UNII: 498M34P1VZ) (OLEA EUROPAEA FLOWER - UNII:498M34P1VZ) OLEA EUROPAEA FLOWER 10 [hp_X] in 59 mL PHOSPHORIC ACID (UNII: E4GA8884NN) (PHOSPHORIC ACID - UNII:E4GA8884NN) PHOSPHORIC ACID 10 [hp_X] in 59 mL SUS SCROFA PITUITARY GLAND (UNII: E8S87O660T) (SUS SCROFA PITUITARY GLAND - UNII:E8S87O660T) SUS SCROFA PITUITARY GLAND 10 [hp_X] in 59 mL SELENIUM (UNII: H6241UJ22B) (SELENIUM - UNII:H6241UJ22B) SELENIUM 10 [hp_X] in 59 mL SEPIA OFFICINALIS JUICE (UNII: QDL83WN8C2) (SEPIA OFFICINALIS JUICE - UNII:QDL83WN8C2) SEPIA OFFICINALIS JUICE 10 [hp_X] in 59 mL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (COLLOIDAL SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 10 [hp_X] in 59 mL THYROID, UNSPECIFIED (UNII: 0B4FDL9I6P) (THYROID, UNSPECIFIED - UNII:0B4FDL9I6P) THYROID, UNSPECIFIED 10 [hp_X] in 59 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57955-0981-2 59 mL in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/10/2014 Labeler - King Bio Inc. (617901350) Registrant - King Bio Inc. (617901350) Establishment Name Address ID/FEI Business Operations King Bio Inc. 617901350 manufacture(57955-0981)