Label: LIP BALM VANILLA FLAVORED- spf 30 lipstick
-
Contains inactivated NDC Code(s)
NDC Code(s): 70445-165-01 - Packager: Webb Business Promotions
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 4, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
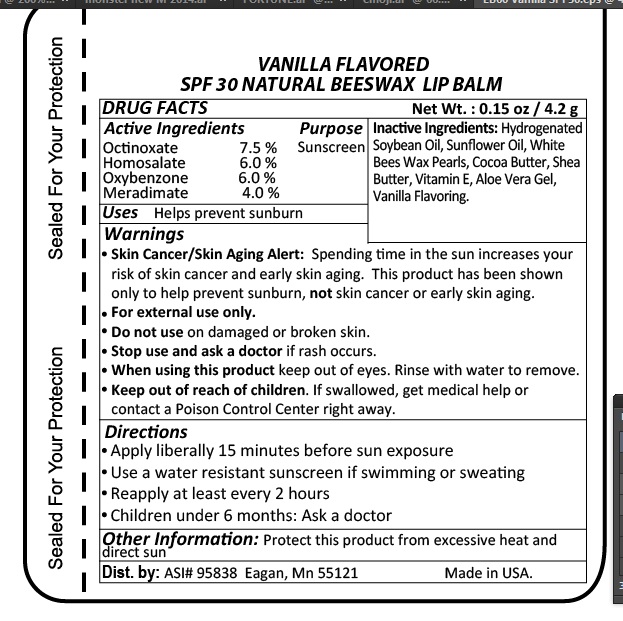
- Purpose
- Drug Facts
- Inactive Ingredients
-
Warnings
- Skin Cancer/Skin Aging Alert: Spending too much time in the sun increases your risk of skin cancer and early skin aging. This product has been shown to only help prevent sunburn, not skin cancer or early skin aging.
- For external use only.
- Do not use on damaged or broken skin.
- Stop use and ask doctor if rash occurs.
- When using this product keep out of eyes.
- Keep out of reach of children. If swallowed get medical help or contact posion control center right away.
- Directions
- Storage and Handling Information
- SPF 30 NATURAL BEESWAX LIP BALM VANILLA FLAVORED Dist. by ASI#95838 Eagan Mn 55121 Made in USA
-
INGREDIENTS AND APPEARANCE
LIP BALM VANILLA FLAVORED
spf 30 lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70445-165 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 6 g in 100 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 g in 100 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 6 g in 100 g MERADIMATE (UNII: J9QGD60OUZ) (MERADIMATE - UNII:J9QGD60OUZ) MERADIMATE 4 g in 100 g Inactive Ingredients Ingredient Name Strength WHITE WAX (UNII: 7G1J5DA97F) SUNFLOWER OIL (UNII: 3W1JG795YI) HYDROGENATED SOYBEAN OIL (UNII: A2M91M918C) COCOA BUTTER (UNII: 512OYT1CRR) SHEA BUTTER (UNII: K49155WL9Y) ALOE VERA LEAF (UNII: ZY81Z83H0X) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70445-165-01 5 g in 1 TUBE; Type 0: Not a Combination Product 09/11/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 01/01/2009 Labeler - Webb Business Promotions (154445647) Establishment Name Address ID/FEI Business Operations Webb Business Promotions 154445647 manufacture(70445-165)