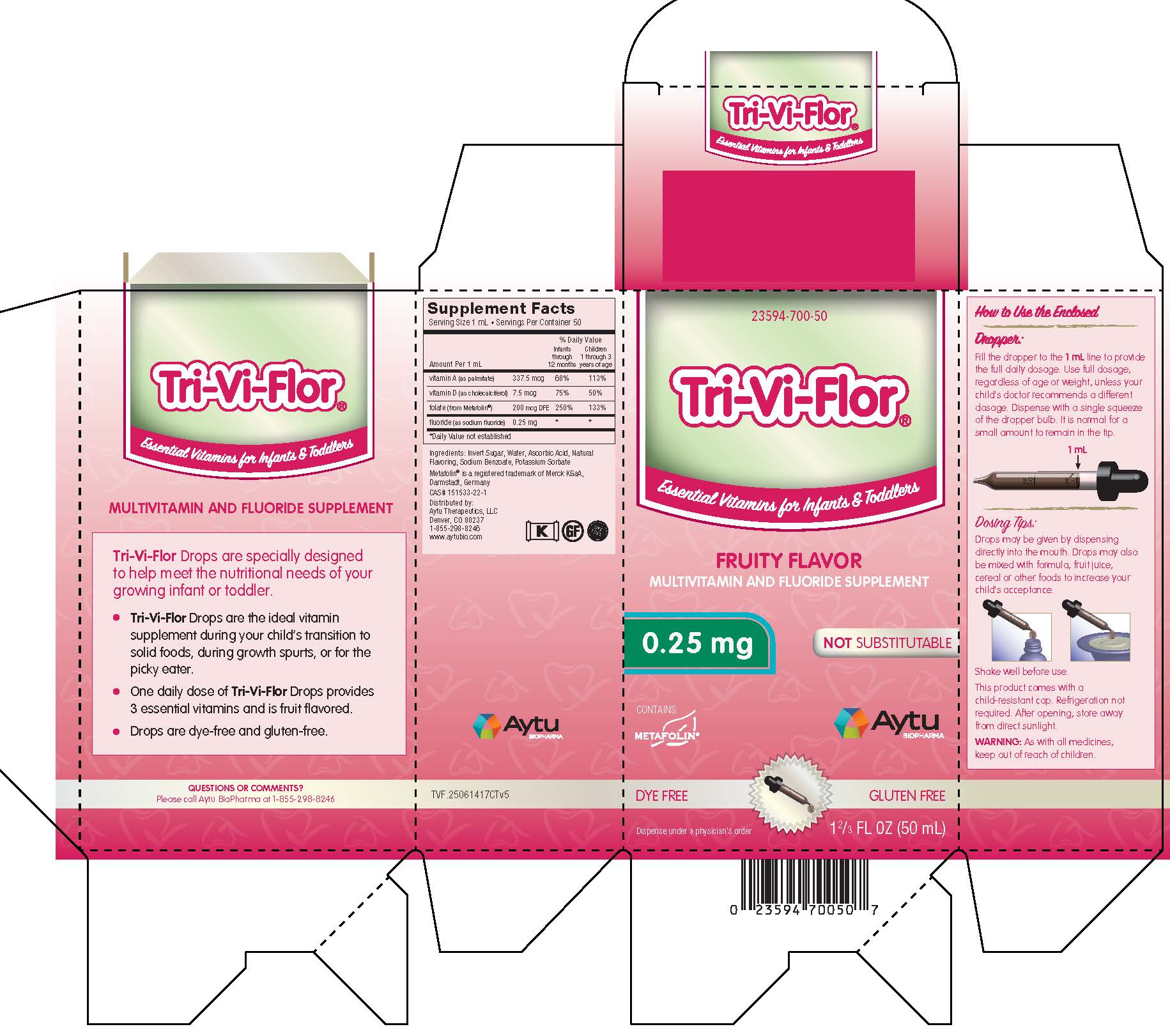
Label: TRI-VI-FLOR- multivitamin and fluoride supplement suspension/ drops
- NHRIC Code(s): 23594-700-50
- Packager: Aytu Therapeutics LLC
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated September 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Tri-Vi-Flor
-
Health Claim
Multivitamin and Fluoride Supplement
Supplement Facts
Serving Size 1 mL • Servings Per Container 50
% Daily Value Amount Per 1 mL
Infants
through
12 monthsChildren
1 through 3
years of agevitamin A (as palmitate) 337.5 mcg 68% 113% vitamin D (as cholecalciferol) 7.5 mcg 75% 50% folate (from Metafolin®) 200 mcg DFE 250% 153% fluoride (as sodium fluoride) 0.25 mg * * *Daily Value not established
Ingredients: Invert Sugar, Water, Ascorbic Acid, Natural Flavoring, Potassium Sorbate
Metafolin® is a registered trademark of Merck KGaA, Darmstadt, Germany
Distributed by: Aytu Therapeutics, LLC
Denver, CO 80237
1-855-298-8246
www.aytubio.com -
Dosage and Administration
How to Use the Enclosed
Dropper:
Fill the dropper to the 1 mL line to provide the full daily dosage. Use full dosage, regardless of age or weight, unless your child's doctor recommends a different dosage. Dispense with a single squeeze of the dropper bulb. It is normal for a small amount to remain in the tip.
Dosing Tips:
Drops may be given by dispensing directly into the mouth. Drops may also be mixed with formula, fruit juice, cereal or other foods to increase your child's acceptance.
Shake well before use.
This product comes with a child-resistant cap. Refrigeration not required. After opening, store away from direct sunlight.
- Warnings
- PRINCIPAL DISPLAY PANEL - Tri-Vi-Flor with 0.25 mg of Fluoride
-
INGREDIENTS AND APPEARANCE
TRI-VI-FLOR
multivitamin and fluoride supplement suspension/ dropsProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:23594-700 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) (VITAMIN A - UNII:81G40H8B0T) VITAMIN A 337.5 ug in 1 mL CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 7.5 ug in 1 mL LEVOMEFOLATE CALCIUM (UNII: A9R10K3F2F) (LEVOMEFOLIC ACID - UNII:8S95DH25XC) LEVOMEFOLIC ACID 200 ug in 1 mL SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.25 mg in 1 mL Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) WATER (UNII: 059QF0KO0R) ASCORBIC ACID (UNII: PQ6CK8PD0R) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:23594-700-50 1 in 1 PACKAGE 1 50 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 09/01/2016 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color flavor Labeler - Aytu Therapeutics LLC (117244733)