Label: PCA SKIN SKIN PROCEDURE- petrolatum ointment
- NDC Code(s): 68726-690-01
- Packager: Colgate Palmolive Skin Health Group, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 24, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
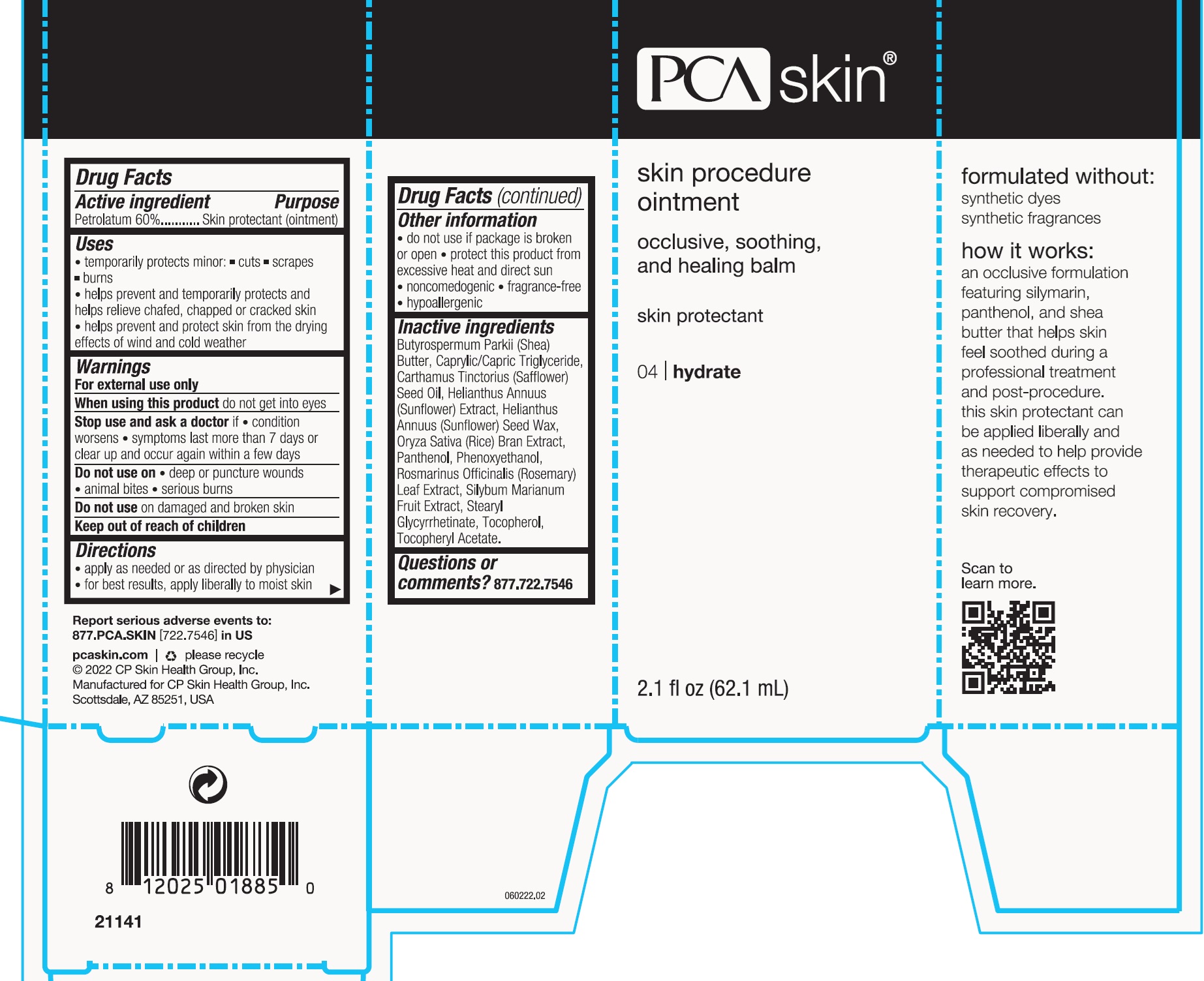
- Drug Facts
-
Active ingredient
Petrolatum 60% Purpose - Skin protectant (ointment)
-
Uses
temporarily protects minor: • cuts • scrapes • burns - helps prevent and temporarily protects and helps relieve chafed, chapped or cracked skin - helps prevent and protect skin from the drying ...
-
Warnings
For external use only - When using this product - do not get into eyes - Stop use and ask a doctor if - condition worsens - symptoms last more than 7 days or clear up and occur again within a few ...
-
Directions
apply as needed or as directed by physician - for best results, apply liberally to moist skin
-
Other information
do not use if package is broken or open - protect this product from excessive heat and direct sun - noncomedogenic - fragrance-free - hypoallergenic
-
Inactive ingredients
Butyrospermum Parkii (Shea) Butter, Caprylic/Capric Triglyceride, Carthamus Tinctorius (Safflower) Seed Oil, Helianthus Annuus (Sunflower) Extract, Helianthus Annuus (Sunflower) Seed Wax, Oryza ...
-
Questions or comments?
877.722.7546
-
Package Labeling:
 ...
... -
INGREDIENTS AND APPEARANCEProduct Information



