Label: GASALIA- activated charcoal, lycopodium clavatum spore, nutmeg, daikon tablet
- NDC Code(s): 0220-9134-04
- Packager: Laboratoires Boiron
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated May 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
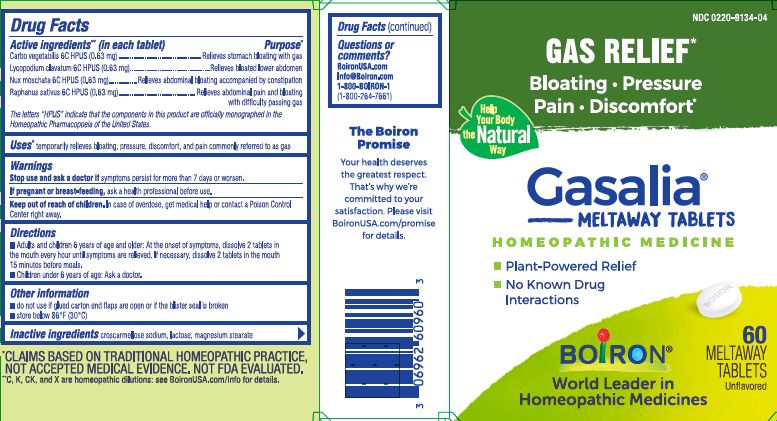
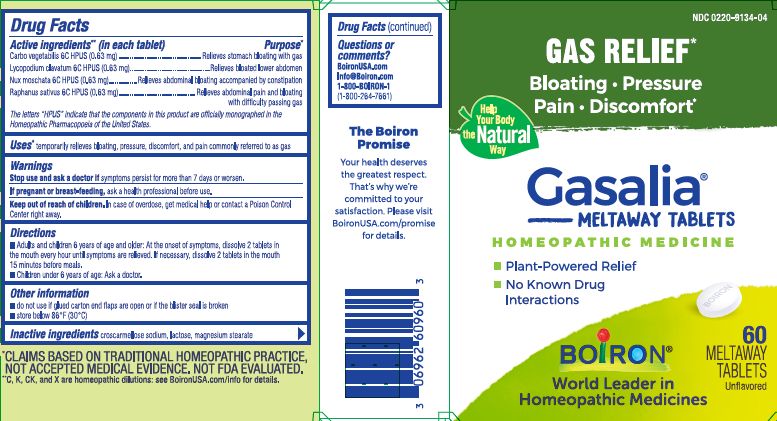
ACTIVE INGREDIENT
Active ingredients** (in each tablet)
Carbo vegetabilis 6C HPUS (0.63 mg)
Lycopodium clavatum 6C HPUS (0.63 mg)
Nux moschata 6C HPUS (0.63 mg)
Raphanus sativus 6C HPUS (0.6 mg)
The letters “HPUS” indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States.
-
PURPOSE
Purpose*
Carbo vegetabilis 6C HPUS ...Relieves stomach bloating with gas
Lycopodium clavatum 6C HPUS ... Relieves bloated lower abdomen
Nux moschata 6C HPUS... Relieves abdominal bloating accompanied by constipation
Raphanus sativus 6C HPUS... Relieves abdominal pain and bloating with difficulty passing gas - INDICATIONS & USAGE
- STOP USE
- WARNINGS
- WHEN USING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
-
SPL UNCLASSIFIED SECTION
do not use if glued carton and flaps are open or if the blister seal is broken.
store below 86°F (30° C)
Plant-Powered Relief
No Known Drug Interactions
60 Meltaway Tablets
*CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE, NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
**C, K, CK, and X are homeopathic dilutions: see BoironUSA.com/info for details.
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GASALIA
activated charcoal, lycopodium clavatum spore, nutmeg, daikon tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0220-9134 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) (ACTIVATED CHARCOAL - UNII:2P3VWU3H10) ACTIVATED CHARCOAL 6 [hp_C] LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 6 [hp_C] NUTMEG (UNII: AEE24M3MQ9) (NUTMEG - UNII:AEE24M3MQ9) NUTMEG 6 [hp_C] DAIKON (UNII: 86R5J6D01D) (DAIKON - UNII:86R5J6D01D) DAIKON 6 [hp_C] Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white Score no score Shape ROUND (BOIRON) Size 9mm Flavor Imprint Code ; Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0220-9134-04 60 in 1 BLISTER PACK; Type 0: Not a Combination Product 08/05/1997 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/05/1997 Labeler - Laboratoires Boiron (282560473) Registrant - Boiron Inc. (014892269) Establishment Name Address ID/FEI Business Operations Boiron 282560473 manufacture(0220-9134)