Label: NIZORAL PSORIASIS RELIEF- salicylic acid cream
- NDC Code(s): 55505-224-33
- Packager: Kramer Laboratories
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- Uses
- WARNINGS
- Ask a doctor before use if
- When using this product
- Stop use and ask doctor if
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
-
Inactive ingredients
allantoin, arachidyl alcohol, arachidyl glucoside, beeswax, behenyl alcohol, benzyl alcohol, butyrospermum parkii(shea) butter, cetearyl alcohol, chlorphenesin, cyclopentasiloxane, dimethicone, disodium EDTA, ethylhexyl isononanoate, glyceryl stearate, hydrogenated polydecene, isododecane, isosorbide dicaprylate, laureth-4, laureth-23, panthenol, PEG-100 stearate, PEG-7 trimethylolpropane coconut ether, propylene glycol, sodium hydroxide, tocopheryl acetate, water, xanthan gum
- QUESTIONS
-
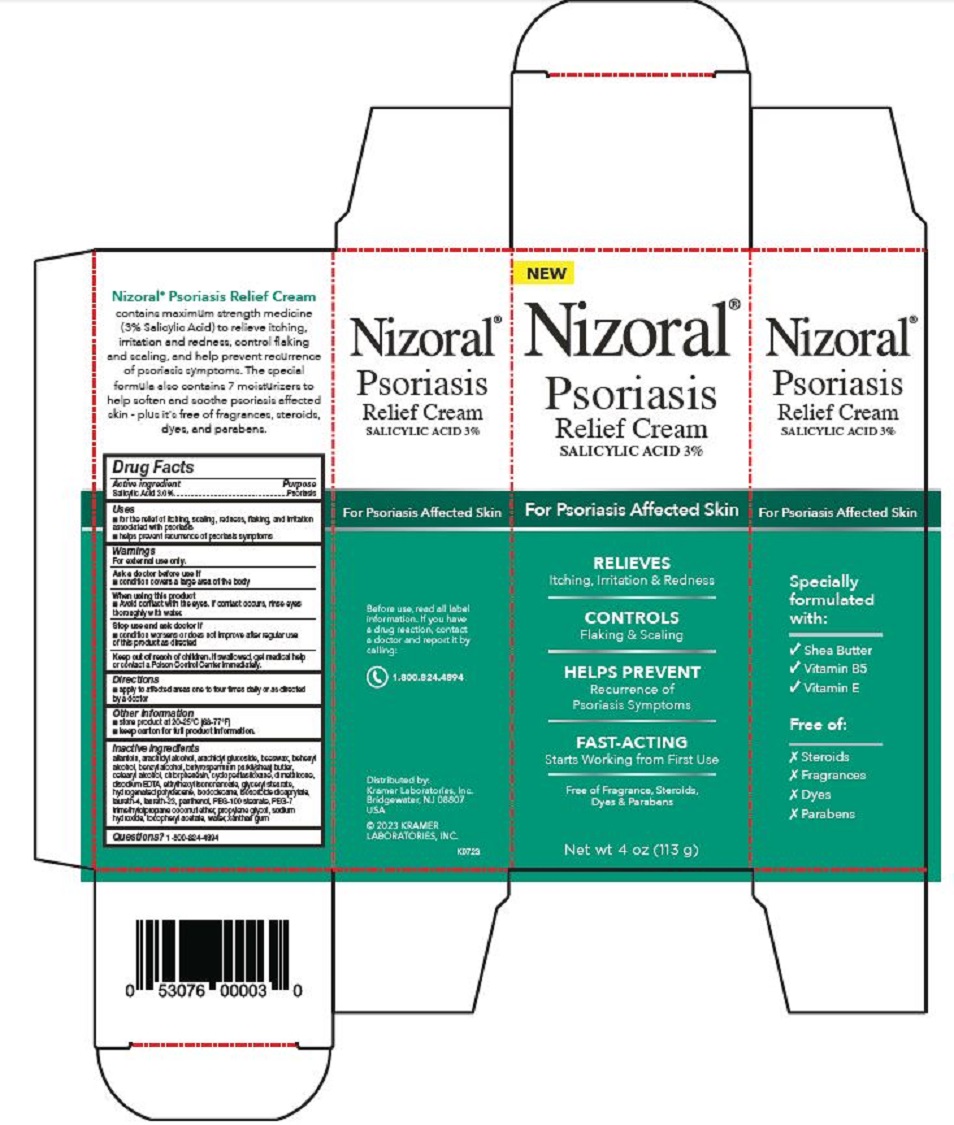
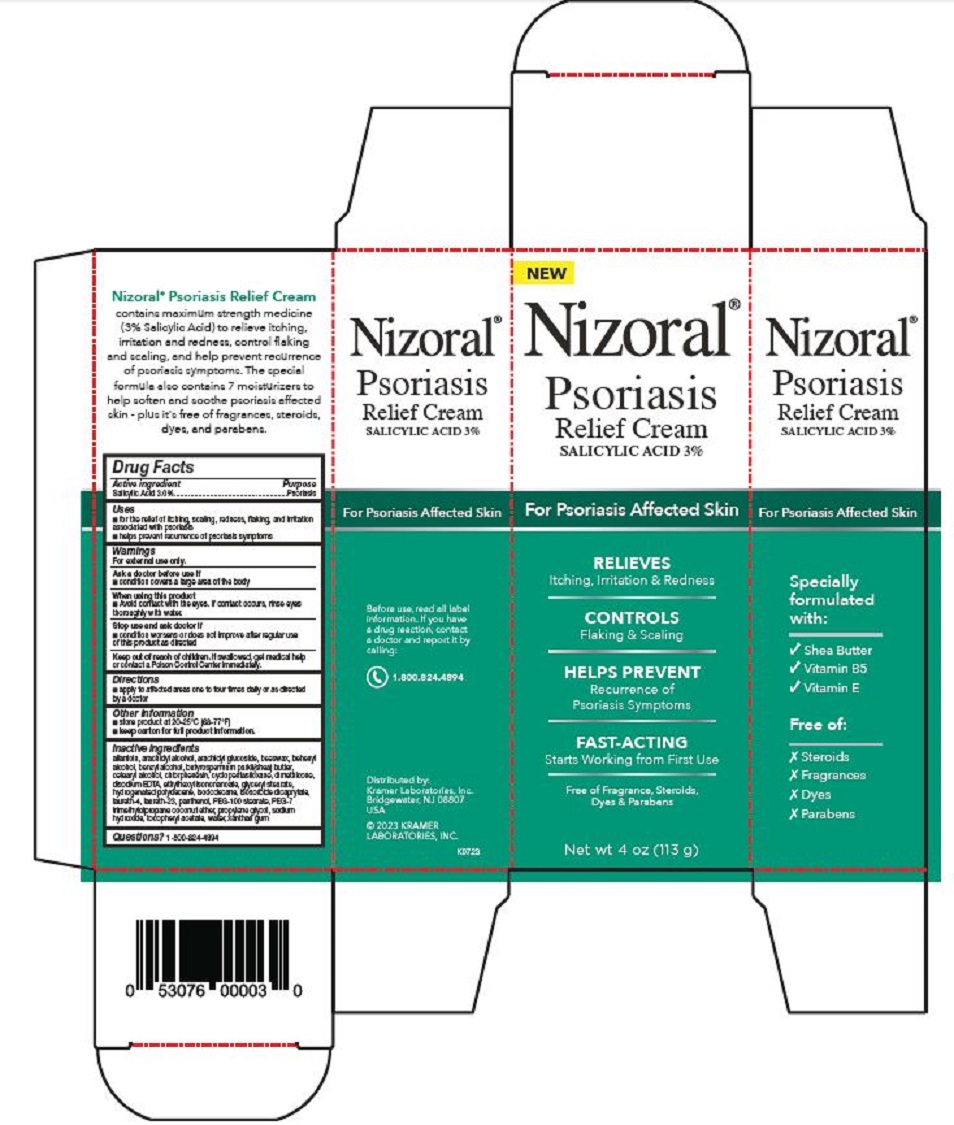
PRINCIPAL DISPLAY PANEL
NEW
Nizoral®
Psoriasis
Relief Cream
SALICYLIC ACID 3%
For Psoriasis Affected Skin
RELIEVES
Itching, Irritation & Redness
CONTROLS
Flaking & Scaling
HELPS PREVENT
Recurrence of
Psoriasis Symptoms
FAST-ACTING
Starts Working from First Use
Free of Fragrance, Steroids,
Dyes & Parabens
Net wt 4 oz (113 g)
Specially
formulated
with:
√ Shea Butter
√ Vitamin B5
√ Vitamin E
Free of:
Ⅹ Steroids
Ⅹ Fragrances
Ⅹ Dyes
Ⅹ Parabens
Before use, read all label
information. If you have
a drug reaction, contact
a doctor and report it by
calling:
1.800.824.4894
Distributed by:
Kramer Laboratories, Inc.
Bridgewater, NJ 08807
USA
© 2023 KRAMER
LABORATORIES, INC.
K0723
-
INGREDIENTS AND APPEARANCE
NIZORAL PSORIASIS RELIEF
salicylic acid creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55505-224 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Salicylic Acid (UNII: O414PZ4LPZ) (Salicylic Acid - UNII:O414PZ4LPZ) Salicylic Acid 3 g in 100 g Inactive Ingredients Ingredient Name Strength Allantoin (UNII: 344S277G0Z) Arachidyl Alcohol (UNII: 1QR1QRA9BU) Arachidyl Glucoside (UNII: 6JVW35JOOJ) Yellow Wax (UNII: 2ZA36H0S2V) Benzyl Alcohol (UNII: LKG8494WBH) Shea Butter (UNII: K49155WL9Y) Cetostearyl Alcohol (UNII: 2DMT128M1S) Chlorphenesin (UNII: I670DAL4SZ) Cyclomethicone 5 (UNII: 0THT5PCI0R) Dimethicone (UNII: 92RU3N3Y1O) Disodium Edta-Copper (UNII: 6V475AX06U) Edetate Disodium Anhydrous (UNII: 8NLQ36F6MM) Glyceryl Stearate Citrate (UNII: WH8T92A065) Hydrogenated Polydecene Type I (UNII: U333RI6EB7) Isododecane (UNII: A8289P68Y2) Isosorbide Dicaprylate (UNII: 0IK29C4889) Laureth-4 (UNII: 6HQ855798J) Laureth-23 (UNII: N72LMW566G) Panthenol (UNII: WV9CM0O67Z) Peg-100 Stearate (UNII: YD01N1999R) Peg-7 Trimethylolpropane Coconut Ether (UNII: MVJ3AD73GG) Propylene Glycol (UNII: 6DC9Q167V3) Sodium Hydroxide (UNII: 55X04QC32I) .Alpha.-Tocopherol Acetate (UNII: 9E8X80D2L0) Water (UNII: 059QF0KO0R) Xanthan Gum (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55505-224-33 1 in 1 CARTON 01/01/2024 1 113 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 01/01/2024 Labeler - Kramer Laboratories (122720675)