Label: CYCLEGUARD 500- melengestrol acetate liquid
- NDC Code(s): 23243-0068-1
- Packager: Huvepharma, Inc
- Category: OTC TYPE A MEDICATED ARTICLE ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated May 12, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
CycleGuard® 500
(melengestrol acetate Type A liquid medicated article)
Heifers Fed in Confinement for Slaughter:
For Increased Rate of Weight Gain, Improved Feed Efficiency and Suppression of Estrus (Heat).Heifers Intended for Breeding: For Suppression of Estrus (Heat).
Each Pound Contains:
Active Drug Ingredient:
Melengestrol Acetate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 500 mg
(as melengestrol acetate and its propylene glycol ketal)
Inactive Ingredient:
Propylene Glycol, U.S.P. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 99.89% - PRECAUTIONS
- STORAGE AND HANDLING
-
INDICATIONS & USAGE
DIRECTIONS FOR USE:
Heifers Fed in Confinement for Slaughter:
CycleGuard 500 (melengestrol acetate Type A liquid medicated article) should be thoroughly
mixed in liquid Type C medicated feed which must be fed at 0.5 to 2.0 pounds per head daily
to provide 0.25 to 0.5 mg of melengestrol acetate per head per day. Average daily intakes
approximating the middle of this range provide the most optimal and economical improvements
in rate of gain and feed utilization. Constant daily intakes of 0.35 to 0.50 mg per head per
day give a high degree of estrus suppression. Levels of 0.25 to 0.35 mg provide a lower but
still effective degree of estrus suppression.
Heifers Intended for Breeding:
CycleGuard 500 should be thoroughly mixed in the supplement to provide 0.5 mg of melengestrol
acetate per head per day. - WARNINGS AND PRECAUTIONS
-
INDICATIONS & USAGE
Heifers Fed in Confinement for Slaughter:
Withdrawal periods of three to five days or more should be avoided to prevent the
possibility that the heifers may come into estrus (heat) at loading time.
Heifers Intended for Breeding:
Do not exceed 24 days of feeding of melengestrol acetate to heifers intended for
breeding. A reduced conception rate can be expected if heifers are bred at estruses
observed within 1 to 12 days after withdrawal of melengestrol acetate, whereas heifers
bred at subsequent observed estruses are expected to have normal conception rates. -
DOSAGE & ADMINISTRATION
MIXING DIRECTIONS:
Liquid Type B and C medicated feeds containing melengestrol acetate must have
a pH of 4.0 to 8.0 and their labels must bear appropriate mixing directions.
Mixing directions for liquid Type B or C feeds stored in recirculation tank
systems are: "Recirculate immediately prior to use for no less than 10 minutes,
moving not less than 1 percent of the tank contents per minute from the bottom
of the tank to the top. Recirculate daily, as directed in this paragraph even
when the Type B (or C) feed is not used." Mixing directions for liquid Type B
and C feeds stored in mechanical, air or other agitation-type tank systems are:
"Agitate immediately prior to use for not less than 10 minutes, creating a
turbulence at the bottom of the tank that is visible at the top. Agitate daily,
as directed in this paragraph, even when the Type B (or C) feed is not used."
Intermediate medicated feeds should not be made from CycleGuard 500 except as
a part of a continuous mixing operation to make a complete liquid Type B or
Type C medicated feed.
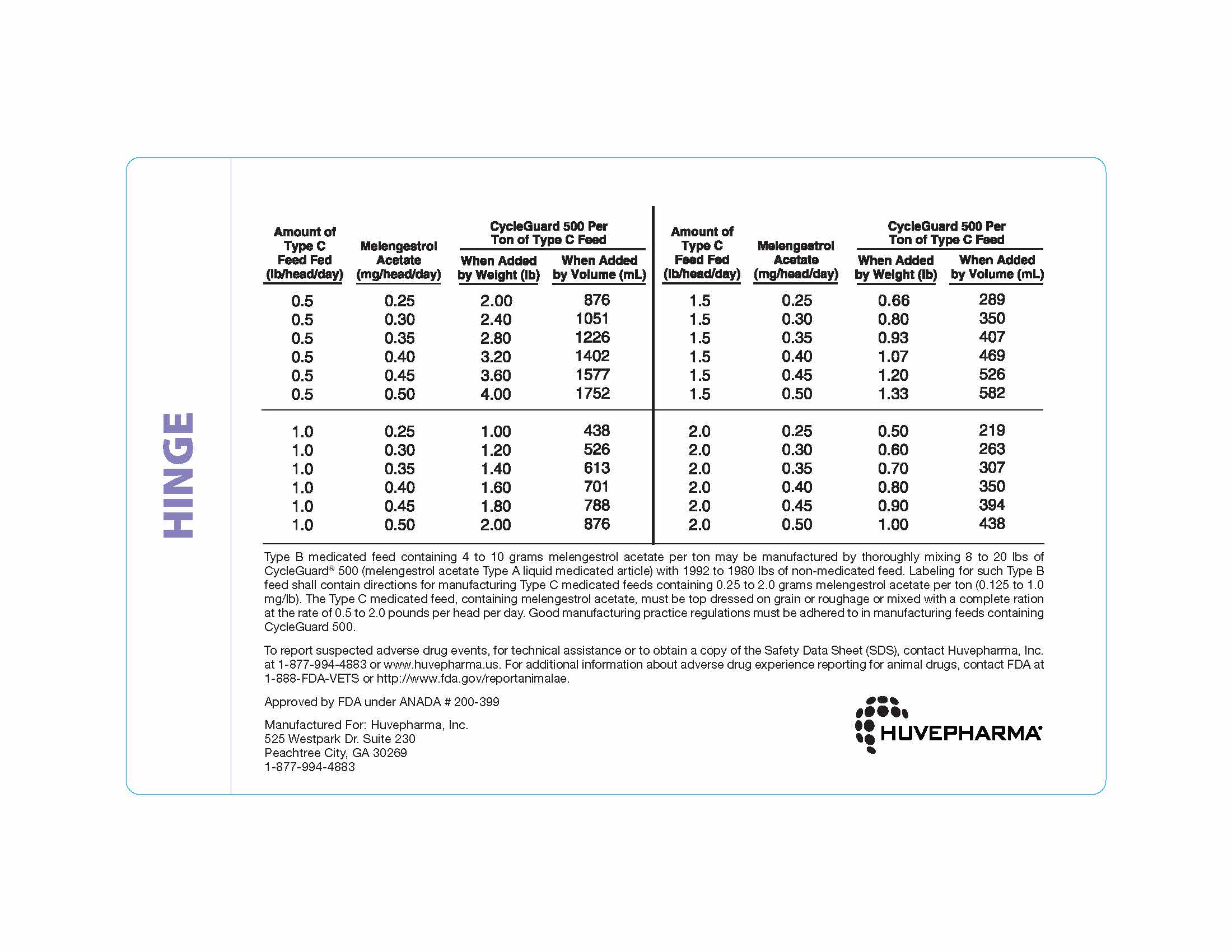
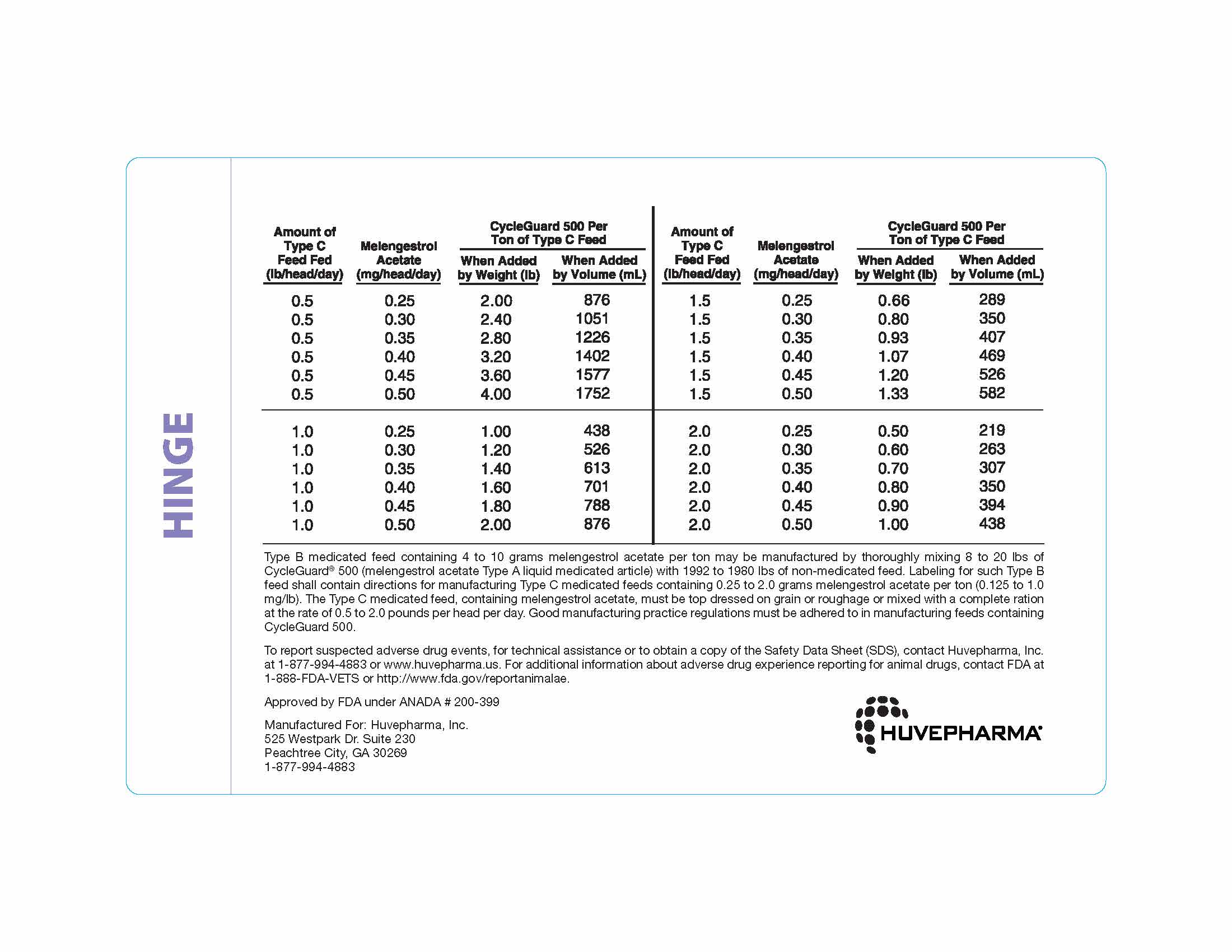
Thoroughly mix 0.5 to 4 pounds of CycleGuard 500 per ton of a non-medicated
feed to prepare a Type C medicated feed containing 0.25 to 2.0 grams of
melengestrol acetate per ton. The following Table may be used as a guide
in determining the amount of CycleGuard 500 to be added to prepare a ton
of Type C medicated feed. -
DOSAGE & ADMINISTRATION
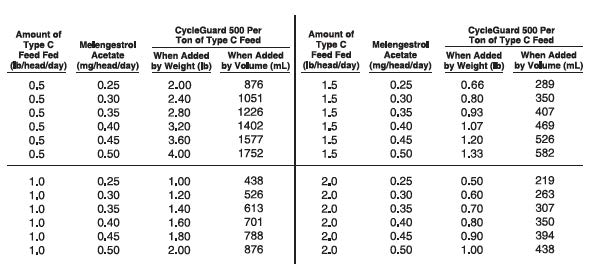
Type B medicated feed containing 4 to 10 grams melengestrol acetate per ton may
be manufactured by thoroughly mixing 8 to 20 lbs of CycleGuard® 500 (melengestrol
acetate Type A liquid medicated article) with 1992 to 1980 lbs of non-medicated
feed. Labeling for such Type B feed shall contain directions for manufacturing
Type C medicated feeds containing 0.25 to 2.0 grams melengestrol acetate per ton
(0.125 to 1.0 mg/lb). The Type C medicated feed, containing melengestrol acetate,
must be top dressed on grain or roughage or mixed with a complete ration at the
rate of 0.5 to 2.0 pounds per head per day. Good manufacturing practice regulations
must be adhered to in manufacturing feeds containing CycleGuard 500. -
ADVERSE REACTIONS
To report suspected adverse drug events, for technical assistance or to obtain a copy
of the Safety Data Sheet (SDS), contact Huvepharma, Inc. at 1-877-994-4883 or
www.huvepharma.us. For additional information about adverse drug experience reporting
for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae. - SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CYCLEGUARD 500
melengestrol acetate liquidProduct Information Product Type OTC TYPE A MEDICATED ARTICLE ANIMAL DRUG Item Code (Source) NDC:23243-0068 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MELENGESTROL ACETATE (UNII: 4W5HDS3936) (MELENGESTROL - UNII:BX98J4T6JU) MELENGESTROL ACETATE 500 mg in .45 kg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:23243-0068-1 18 kg in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200399 02/21/2021 Labeler - Huvepharma, Inc (619153559)